As noted on several previous occasions, isomers are compounds with the same chemical formula but different structures. We’ve seen several kinds of isomers in the past few chapters, and it’s a good idea at this point to see how they relate to one another (Figure 5.15).
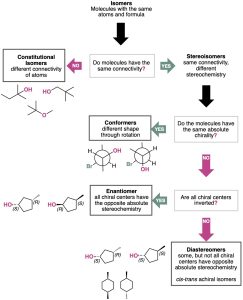
Figure 5.15 A summary of the different kinds of isomers.
There are two fundamental types of isomers, both of which we’ve now encountered: constitutional isomers and stereoisomers.
Constitutional isomers (Section 3.2) are compounds whose atoms are connected differently. For example, 2-methylpropane and butane are constitutional isomers. Other examples of constitutional isomers are ethanol and dimethyl ether; and isopropylamine and propylamine.
Practice
Try drawing the structures of isopropylamine and propylamine. Confirm they have the same exact chemical formula but different connectivity of atoms.
Stereoisomers (Section 4.2) are compounds whose atoms are connected in the same order but with a different spatial arrangement. Among the kinds of stereoisomers we’ve seen are conformational isomers (conformers), as well as enantiomers, diastereomers. It is helpful to note that two molecules that are enantiomers must be chiral, non-superimposable mirror images of one another. However, diastereomers can be chiral (e.g. some chiral centers have different configuration) or achiral (e.g. cis–trans isomers).
Problem 5-21
What kinds of isomers are the following pairs?
(a) (S)-5-chloro-2-hexene and chlorocyclohexane (b) (2R,3R)-dibromopentane and (2S,3R)-dibromopentane

