SN1, SN2, E1, E1cB, E2—how can you keep it all straight and predict what will happen in any given case? Will substitution or elimination occur? Will the reaction be bimolecular or unimolecular? There are no rigid answers to these questions, but it’s possible to recognize some trends and make some generalizations.
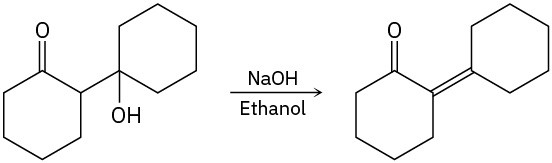
- Primary alkyl halides. SN2 substitution occurs if a good nucleophile is used, E2 elimination occurs if a strong, sterically hindered base is used, and E1cB elimination occurs if the leaving group is two carbons away from a carbonyl group.
- Secondary alkyl halides. SN2 substitution occurs if a weakly basic nucleophile is used in a polar aprotic solvent, E2 elimination predominates if a strong base is used, and E1cB elimination takes place if the leaving group is two carbons away from a carbonyl group. Secondary allylic and benzylic alkyl halides can also undergo SN1 and E1 reactions if a weakly basic nucleophile is used in a protic solvent.
- Tertiary alkyl halides. E2 elimination occurs when a base is used, but SN1 substitution and E1 elimination occur together under neutral conditions, such as in pure ethanol or water. E1cB elimination takes place if the leaving group is two carbons away from a carbonyl group.
Worked Example 11.5 Predicting the Product and Mechanism of Reactions
Tell whether each of the following reactions is likely to be SN1, SN2, E1, E1cB, or E2, and predict the product of each:

Strategy
Look carefully in each reaction at the structure of the substrate, the leaving group, the nucleophile, and the solvent. Then decide from the preceding summary which kind of reaction is likely to be favored.
Solution
(a) A secondary, nonallylic substrate can undergo an SN2 reaction with a good nucleophile in a polar aprotic solvent but will undergo an E2 reaction on treatment with a strong base in a protic solvent. In this case, E2 reaction is likely to predominate.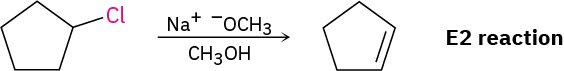
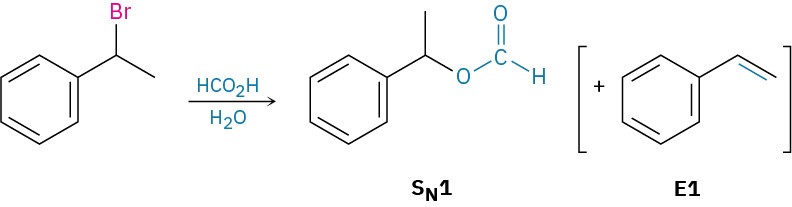
(b) A secondary benzylic substrate can undergo an SN2 reaction on treatment with a nonbasic nucleophile in a polar aprotic solvent and will undergo an E2 reaction on treatment with a base. Under protic conditions, such as aqueous formic acid (HCO2H), an SN1 reaction is likely, along with some E1 reaction.
Problem 11-20
Tell whether each of the following reactions is likely to be SN1, SN2, E1, E1cB, or E2:
(a)
![]()
(b)
![]()
(c)
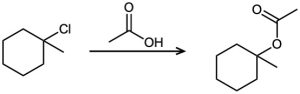
(d)