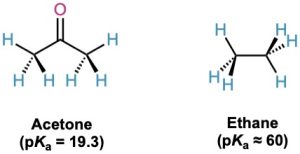
As noted in Section 22.1, a hydrogen on the α position of a carbonyl compound is weakly acidic and can be removed by a strong base to yield an enolate ion. In comparing acetone (pKa = 19.3) with ethane (pKa ≈ 60), for instance, the presence of a neighboring carbonyl group increases the acidity of the ketone over the alkane by a factor of 1040.
Proton abstraction from a carbonyl compound occurs when the α C–H bond is oriented roughly parallel to the p orbitals of the carbonyl group. The α carbon atom of the enolate ion is sp2-hybridized and has a p orbital that overlaps the neighboring carbonyl p orbitals. Thus, the negative charge is shared by the electronegative oxygen atom, and the enolate ion is stabilized by resonance (Figure 22.5).
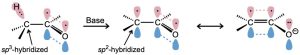
Figure 22.5 Mechanism of enolate-ion formation by abstraction of an α proton from a carbonyl compound. The enolate ion is stabilized by resonance, and the negative charge (red) is shared by the oxygen and the α carbon atom, as indicated in the electrostatic potential map.
Because carbonyl compounds are only weakly acidic, a strong base is needed for the formation of an enolate-ion. If an alkoxide ion, such as sodium ethoxide, is used as base, deprotonation of acetone only takes place to the extent of about 0.1% because acetone is a weaker acid than ethanol (pKa = 16). If, however, a more powerful base is used, then a carbonyl compound is completely converted into its enolate ion.
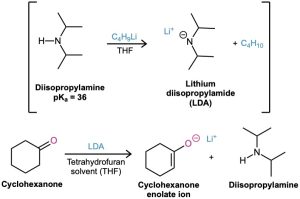
In practice, the strong base lithium diisopropylamide [LiN(i-C3H7)2; abbreviated LDA] is commonly used for making enolate ions. As the lithium salt of the weak acid diisopropylamine, pKa = 36, LDA can readily deprotonate most carbonyl compounds. It is easily prepared by reaction of butyllithium with diisopropylamine and is soluble in organic solvents because of its two alkyl groups.
Many types of carbonyl compounds, including aldehydes, ketones, esters, thioesters, acids, and amides, can be converted into enolate ions by reaction with LDA. Table 22.1 lists the approximate pKa values of different types of carbonyl compounds and shows how these values compare to other acidic substances we’ve seen. Note that nitriles, too, are acidic and can be converted into enolate-like anions.
Table 22.1 Acidity Constants for Some Organic Compounds
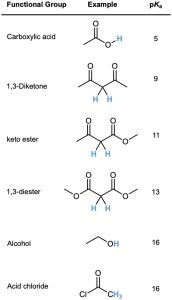
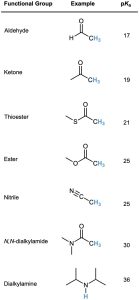
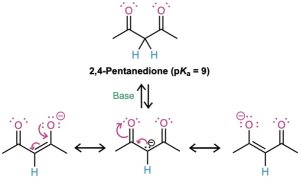
When a hydrogen atom is flanked by two carbonyl groups, its acidity is enhanced further. Table 22.1 thus shows that 1,3-diketones (β-diketones), 3-oxo esters (β-keto esters), and 1,3-diesters (malonic esters) are more acidic than water. The enolate ions derived from these β-dicarbonyl compounds are stabilized by sharing the negative charge of the two neighboring carbonyl oxygens. The enolate ion of 2,4-pentanedione, for instance, has three resonance forms. Similar resonance forms can be drawn for other doubly stabilized enolate ions.
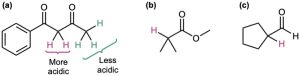
Worked Example 22.1 – Identifying the Acidic Hydrogens in a Compound
Identify the most acidic hydrogens in each of the following compounds, and rank the compounds in order of increasing acidity:

Strategy: Hydrogens on carbon next to a carbonyl group are acidic. In general, a β-dicarbonyl compound is most acidic, a ketone or aldehyde is next most acidic, and a carboxylic acid derivative is least acidic. Remember that alcohols, phenols, and carboxylic acids are also acidic because of their –OH hydrogens.
Solution: The acidity order is (a) > (c) > (b). Acidic hydrogens are shown in red.
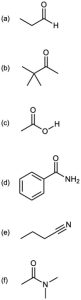
Problem 22-7
Identify the most acidic hydrogens in each of the following molecules:
Problem 22-8
Draw a resonance structure of the acetonitrile anion, –:CH2C≡N, and account for the acidity of nitriles.

