Perhaps the most important of all concepts related to electronegativity and polarity is that of acidity and basicity. We’ll soon see, in fact, that the acid–base behavior of organic molecules explains much of their chemistry. You may recall from a course in general chemistry that two definitions of acidity are frequently used: the Brønsted–Lowry definition and the Lewis definition. We’ll look at the Brønsted–Lowry definition in this and the following three sections and then discuss the Lewis definition in Section 2.11.
A Brønsted–Lowry acid is a substance that donates a proton (H+), and a Brønsted– Lowry base is a substance that accepts a proton. When gaseous hydrogen chloride dissolves in water, for example, a polar HCl molecule acts as an acid and donates a proton, while a water molecule acts as a base and accepts the proton, yielding chloride anion (Cl–) and hydronium cation (H3O+). This and other acid–base reactions are reversible, so we’ll write them with double, forward-and-backward arrows.
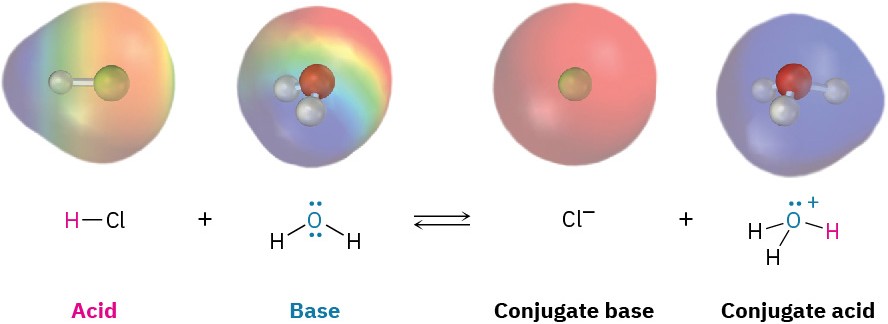
Chloride ion, the product that results when the acid HCl loses a proton, is called the conjugate base of the acid, and hydronium ion, the product that results when the base H2O gains a proton, is called the conjugate acid of the base.
In a general sense,
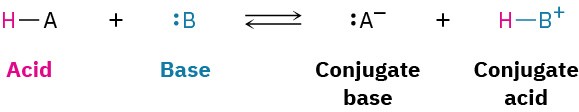
For example:
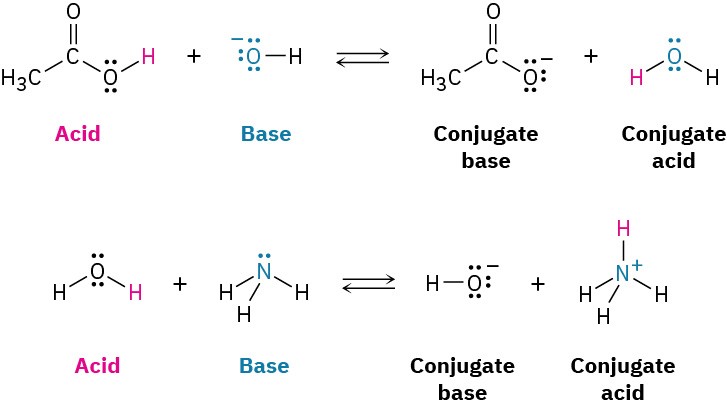
Notice that water can act either as an acid or as a base, depending on the circumstances. In its reaction with HCl, water is a base that accepts a proton to give the hydronium ion, H3O+. In its reaction with ammonia (NH3), however, water is an acid that donates a proton to give ammonium ion (NH4+) and hydroxide ion, HO–.
Problem 2-11
Nitric acid (HNO3) reacts with ammonia (NH3) to yield ammonium nitrate. Write the reaction, and identify the acid, the base, the conjugate acid product, and the conjugate base product.

