Yet another kind of alkene addition is the reaction with a carbene to yield a cyclopropane. A carbene, R2C:, is a neutral molecule containing a divalent carbon with only six electrons in its valence shell. It is therefore highly reactive and generated only as a reaction intermediate, rather than as an isolable molecule. Because they’re electron-deficient, carbenes behave as electrophiles and react with nucleophilic C═C bonds. The reaction occurs in a single step without intermediates.
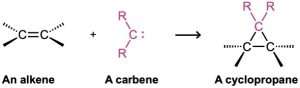
One of the simplest methods for generating a substituted carbene is by treatment of chloroform, CHCl3, with a strong base such as KOH. As shown in Figure 8.10, the loss of a proton from CHCl3 gives trichloromethanide anion, −:CCl2, which spontaneously expels a Cl− ion to yield dichlorocarbene, :CCl2.
Figure 8.10 MECHANISM
Mechanism of the formation of dichlorocarbene by reaction of chloroform with strong base. Deprotonation of CHCl3 gives the trichloromethanide anion,−:CCl2, which spontaneously expels a Cl– ion.
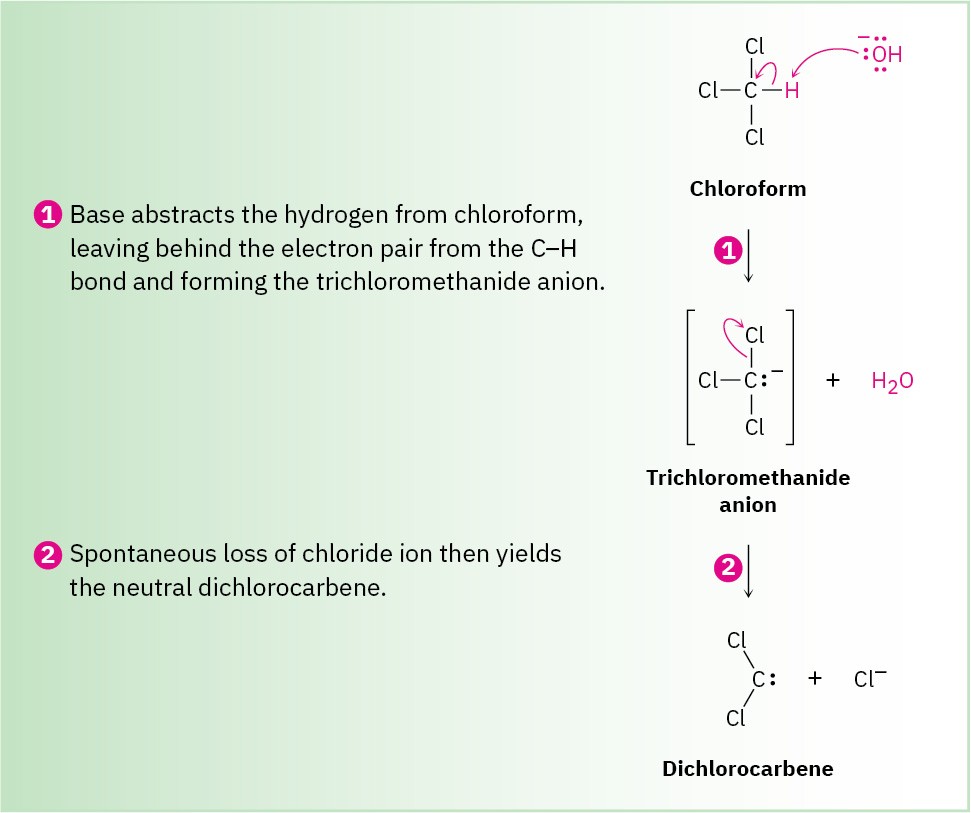
The carbon atom in dichlorocarbene is sp2-hybridized, with a vacant p orbital extending above and below the plane of the three atoms and with an unshared pair of electrons occupying the third sp2 lobe. Note that this electronic description of dichlorocarbene is similar to that of a carbocation (Section 7.9) with respect to both the sp2 hybridization of carbon and the vacant p orbital. Electrostatic potential maps further illustrate the similarity (Figure 8.11).
Figure 8.11 The structure of dichlorocarbene. Electrostatic potential maps show how the positive region coincides with the empty p orbital in both dichlorocarbene and a carbocation (CH3+). The negative region in the dichlorocarbene map coincides with the lone-pair electrons.
If dichlorocarbene is generated in the presence of an alkene, addition to the double bond occurs and a dichlorocyclopropane is formed. As the reaction of dichlorocarbene with cis-2-pentene demonstrates, the addition is stereospecific, meaning that only a single stereoisomer is formed as product. Starting from a cis alkene, for instance, only cis-disubstituted cyclopropane is produced; starting from a trans alkene, only trans- disubstituted cyclopropane is produced.
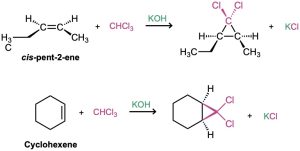
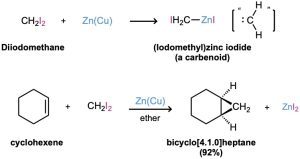
The best method for preparing nonhalogenated cyclopropanes is by a process called the Simmons–Smith reaction. First investigated at the DuPont company, this reaction does not involve a free carbene. Rather, it utilizes a carbenoid—a metal-complexed reagent with carbene-like reactivity. When diiodomethane is treated with a specially prepared zinc–copper mix, (iodomethyl)zinc iodide, ICH2ZnI, is formed. In the presence of an alkene, ICH2ZnI transfers a CH2 group to the double bond to yield cyclopropane. For example, cyclohexene reacts cleanly and with good yield to give the corresponding cyclopropane.
Although we won’t discuss the mechanistic details, carbene addition to an alkene is one of a general class of reactions called cycloadditions, which we’ll study more carefully in Chapter 30.
Problem 8-17
What products would you expect from the following reactions?
(a)
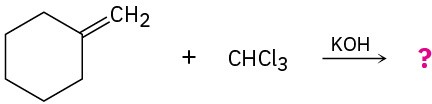
(b)
![]()

