Perhaps the most useful reaction of enolate ions is their alkylation by treatment with an alkyl halide, thereby forming a new C−C bond and joining two smaller pieces into one larger molecule. Alkylation occurs when the nucleophilic enolate ion reacts with the electrophilic alkyl halide in an SN2 reaction and displaces the leaving group by backside attack.
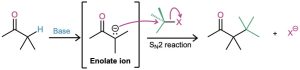
Alkylation reactions are subject to the same constraints that affect all SN2 reactions (Section 11.3). Thus, the leaving group X in the alkylating agent R−X can be chloride, bromide, or iodide. The alkyl group R should be primary or methyl, and preferably should be allylic or benzylic. Secondary halides react poorly, and tertiary halides don’t react at all because a competing E2 elimination of HX occurs instead. Vinylic and aryl halides are also unreactive because a backside approach is sterically prevented.
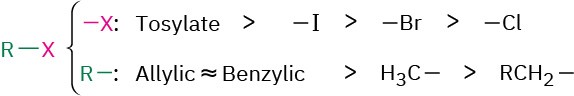
The Malonic Ester Synthesis
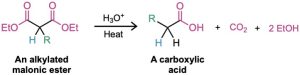
One of the oldest and best known carbonyl alkylation reactions is the malonic ester synthesis, a method for preparing a carboxylic acid from an alkyl halide while lengthening the carbon chain by two atoms.
![]()
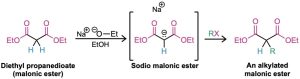
Diethyl propanedioate, commonly called diethyl malonate, or malonic ester, is relatively acidic (pKa = 13) because its α hydrogens are flanked by two carbonyl groups. Thus, malonic ester is easily converted into its enolate ion by reaction with sodium ethoxide in ethanol. The enolate ion, in turn, is a good nucleophile that reacts rapidly with an alkyl halide to give an α-substituted malonic ester. Note in the following examples that the abbreviation “Et” is used for an ethyl group, –CH2CH3.
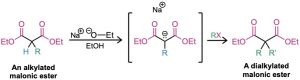
The product of a malonic ester alkylation has one acidic α hydrogen remaining, so the alkylation process can be repeated to yield a dialkylated malonic ester.
On heating with aqueous hydrochloric acid, the alkylated (or dialkylated) malonic ester undergoes hydrolysis of its two ester groups followed by decarboxylation (loss of CO2) to yield a substituted monocarboxylic acid.
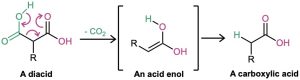
Decarboxylation is not a general reaction of carboxylic acids. Rather, it is unique to compounds that have a second carbonyl group two atoms away from the –CO2H. That is, only substituted malonic acids and β-keto acids undergo loss of CO2 on heating. The decarboxylation reaction occurs by a cyclic mechanism and involves initial formation of an enol, thereby accounting for the need to have a second carbonyl group appropriately positioned.
As noted previously, the overall effect of malonic ester synthesis is to convert an alkyl halide into a carboxylic acid while lengthening the carbon chain by two atoms (RX → RCH2CO2H).
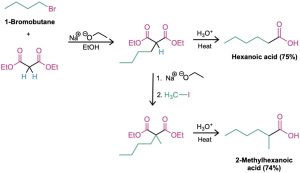
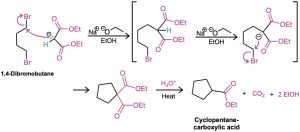
A malonic ester synthesis can also be used to prepare cycloalkanecarboxylic acids. For example, when 1,4-dibromobutane is treated with diethyl malonate in the presence of two equivalents of sodium ethoxide base, the second alkylation step occurs intramolecularly to yield a cyclic product. Hydrolysis and decarboxylation then give cyclopentanecarboxylic acid. Three-, four-, five-, and six-membered rings can be prepared in this way, but yields decrease for larger ring sizes.
Worked Example 22.2- Using Malonic Ester Synthesis to Prepare a Carboxylic Acid
How would you prepare heptanoic acid using a malonic ester synthesis?
Strategy: A malonic ester synthesis converts an alkyl halide into a carboxylic acid having two more carbons. Thus, a seven-carbon acid chain must be derived from the five-carbon alkyl halide 1-bromopentane.
Solution
![]()
Problem 22-10
How could you use a malonic ester synthesis to prepare the following compounds? Show all steps.
(a)
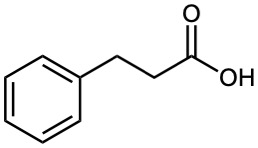
(b)
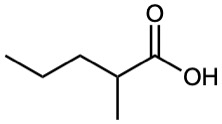
(c)
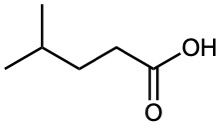
Problem 22-11
Monoalkylated and dialkylated acetic acids can be prepared by malonic ester synthesis, but trialkylated acetic acids (R3C–CO2H) can’t be prepared. Explain.
Problem 22-12
How could you use a malonic ester synthesis to prepare the following compound?
The Acetoacetic Ester Synthesis
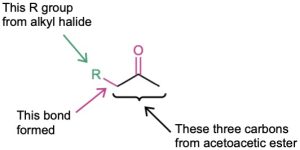
Just as the malonic ester synthesis converts an alkyl halide into a carboxylic acid, the acetoacetic ester synthesis converts an alkyl halide into a methyl ketone having three more carbons.
![]()
Ethyl 3-oxobutanoate, commonly called ethyl acetoacetate, or acetoacetic ester, is much like malonic ester in that its α hydrogens are flanked by two carbonyl groups. It is therefore readily converted into its enolate ion, which can be alkylated by reaction with an alkyl halide. A second alkylation can also be carried out if desired, since acetoacetic ester has two acidic α hydrogens.
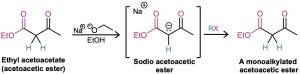
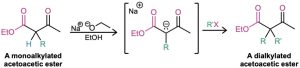
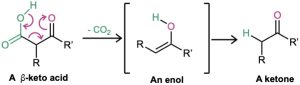
On heating with aqueous HCl, the alkylated (or dialkylated) acetoacetic ester is hydrolyzed to a β-keto acid, which then undergoes decarboxylation to yield a ketone product. The decarboxylation occurs in the same way as in the malonic ester synthesis and involves a ketone enol as the initial product.
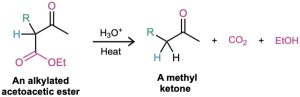
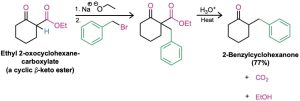
The three-step sequence of (1) enolate ion formation, (2) alkylation, and (3) hydrolysis/decarboxylation is applicable to all β-keto esters with acidic α hydrogens, not just to acetoacetic ester itself. For example, cyclic β-keto esters, such as ethyl 2-oxocyclohexanecarboxylate, can be alkylated and decarboxylated to give 2-substituted cyclohexanones.
Worked Example 22.3– Using Acetoacetic Ester Synthesis to Prepare a Ketone
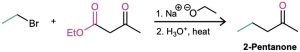
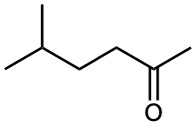
How would you prepare 2-pentanone by an acetoacetic ester synthesis?
Strategy: An acetoacetic ester synthesis yields a methyl ketone by adding three carbons to an alkyl halide.
Thus, the acetoacetic ester synthesis of 2-pentanone must involve reaction of bromoethane.
Solution:
Problem 22-13
What alkyl halides would you use to prepare the following ketones by an acetoacetic ester synthesis?
(a)
(b)
![]()
Problem 22-14
Which of the following compounds can’t be prepared by an acetoacetic ester synthesis? Explain.
(a) Phenylacetone
(b) Acetophenone
(c) 3,3-Dimethyl-2-butanone
Problem 22-15
How would you prepare the following compound using an acetoacetic ester synthesis?
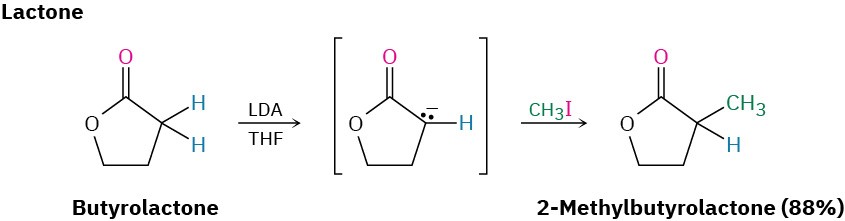
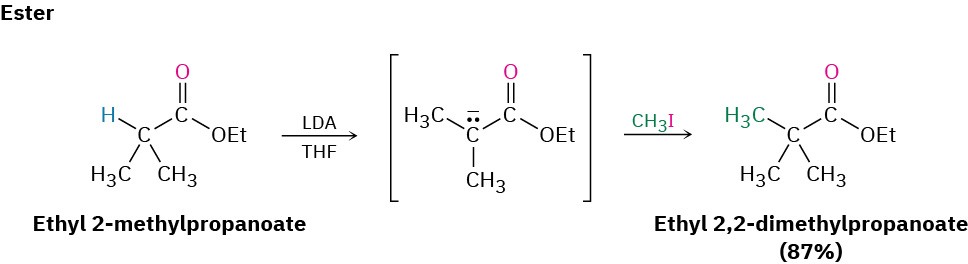
Direct Alkylation of Ketones, Esters, and Nitriles
Both the malonic ester synthesis and the acetoacetic ester synthesis are easy to carry out because they involve relatively acidic dicarbonyl compounds. As a result, sodium ethoxide in ethanol can be used to prepare the necessary enolate ions. Alternatively, however, it’s also possible in many cases to directly alkylate the α position of monocarbonyl compounds. A strong, sterically hindered base such as LDA is needed so that complete conversion to the enolate ion takes place rather than a nucleophilic addition, and a nonprotic solvent must be used.
Ketones, esters, and nitriles can all be alkylated using LDA or related dialkylamide bases in tetrahydrofuran (THF). Aldehydes, however, rarely give high yields of pure products because their enolate ions undergo carbonyl condensation reactions instead of alkylation. (We’ll study this condensation reaction in the next chapter.) Some specific examples of alkylation reactions are shown.
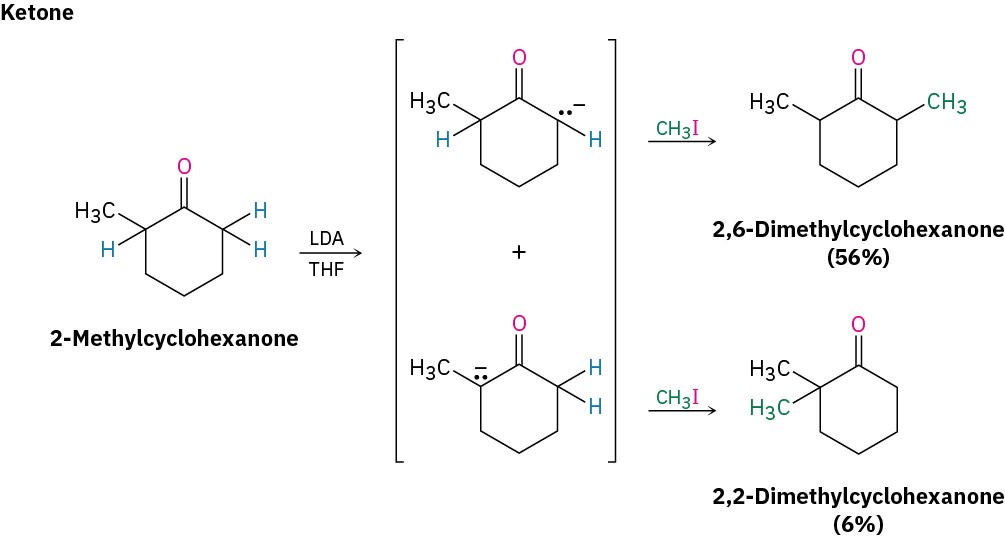
Note in the ketone example that alkylation of 2-methylcyclohexanone leads to a mixture of products because both possible enolate ions are formed. In general, the major product in such cases occurs by alkylation at the less hindered, more accessible position. Thus, alkylation of 2-methylcyclohexanone occurs primarily at C6 (secondary) rather than C2 (tertiary).
Worked Example 22.4 – Using an Alkylation Reaction to Prepare a Substituted Ester
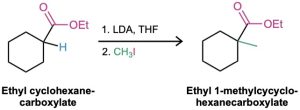
How might you use an alkylation reaction to prepare ethyl 1-methylcyclohexanecarboxylate?

Strategy: An alkylation reaction is used to introduce a methyl or primary alkyl group onto the α position of a ketone, ester, or nitrile by SN2 reaction of an enolate ion with an alkyl halide. Thus, we need to look at the target molecule and identify any methyl or primary alkyl groups attached to an α carbon. In the present instance, the target has an α methyl group, which might be introduced by alkylation of an ester enolate ion with iodomethane.
Solution:
Problem 22-16
Show how you might prepare the following compounds using an alkylation reaction as the key step:
(a)
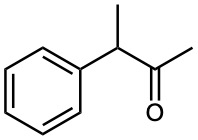
(b)
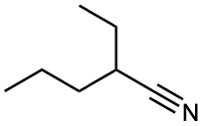
(c)
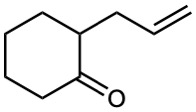
(d)
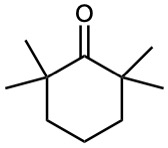
(e)
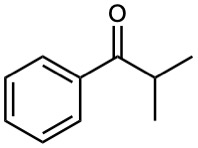
(f)
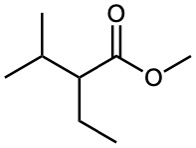
Biological Alkylations
Alkylations are rare but not unknown in biological chemistry. One example occurs during biosynthesis of the antibiotic indolmycin from indolylpyruvate when a base abstracts an acidic hydrogen from an α position and the resultant enolate ion carries out an SN2 alkylation reaction on the methyl group of S-adenosylmethionine (SAM; Section 11.6).
Although it’s convenient to speak of “enolate ion” intermediates in biological pathways, it’s unlikely that they exist for long in an aqueous cellular environment. Rather, proton removal and alkylation probably occur at essentially the same time (Figure 22.7).
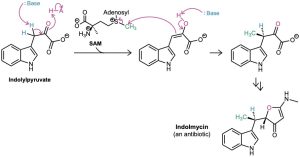
Figure 22.7 The biosynthesis of indolmycin from indolylpyruvate occurs through a pathway that includes an alkylation reaction of a short-lived enolate ion intermediate.

