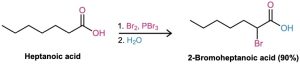
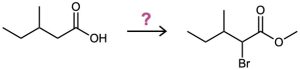The α-bromination of carbonyl compounds by Br2 in acetic acid is limited to aldehydes and ketones because acids, esters, and amides don’t enolize to a sufficient extent. Carboxylic acids, however, can be α-brominated by a mixture of Br2 and PBr3 in the Hell–Volhard– Zelinskii (HVZ) reaction.
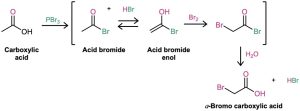
The Hell–Volhard–Zelinskii reaction is a bit more complex than it looks and actually involves α substitution of an acid bromide enol rather than a carboxylic acid enol. The process begins by reaction of the carboxylic acid with PBr3 to form an acid bromide plus HBr (Section 21.4). The HBr then catalyzes enolization of the acid bromide, and the resultant enol reacts with Br2 in an α-substitution reaction to give an α-bromo acid bromide. Addition of water hydrolyzes the acid bromide in a nucleophilic acyl substitution reaction and yields the α-bromo carboxylic acid product.
Problem 22-6
If methanol rather than water is added at the end of a Hell–Volhard–Zelinskii reaction, an ester rather than an acid is produced. Show how you would carry out the following transformation, and propose a mechanism for the ester-forming step.