A particularly common α-substitution reaction in the laboratory is the halogenation of aldehydes and ketones at their α positions by reaction with Cl2, Br2, or I2 in acidic solution. Bromine in acetic acid solvent is often used.
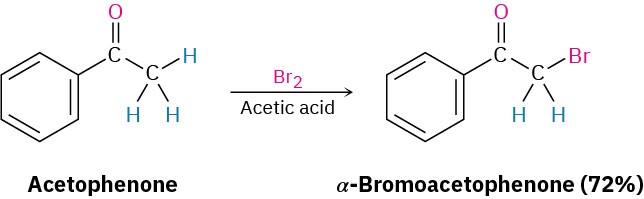
Remarkably, ketone halogenation also occurs in biological systems, particularly in marine alga, where dibromoacetaldehyde, bromoacetone, 1,1,1-tribromoacetone, and other related compounds have been found.
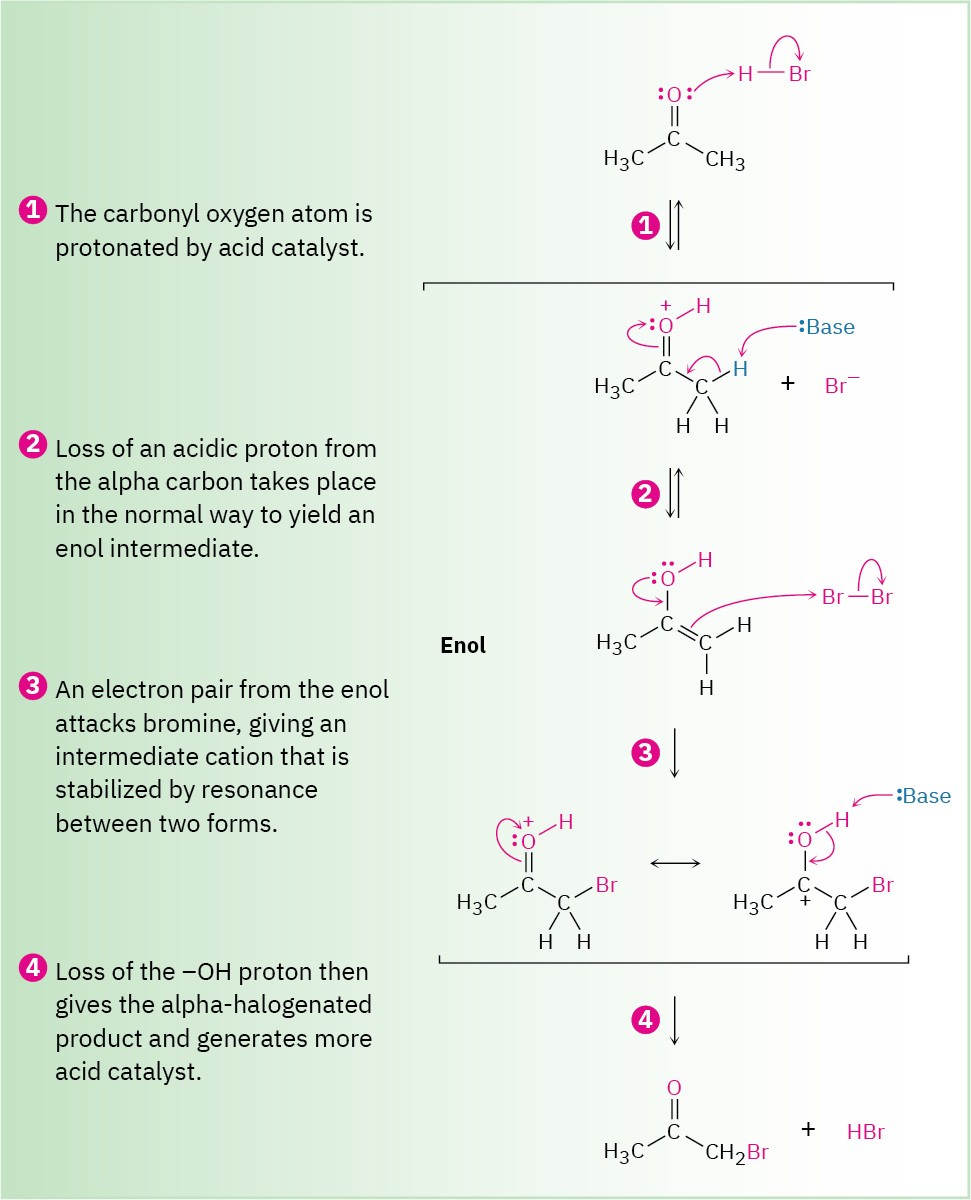
This form of halogenation is a typical α-substitution reaction that proceeds by acid-catalyzed formation of an enol intermediate, as shown in Figure 22.4.
Figure 22.4 Mechanism of the acid-catalyzed bromination of acetone.
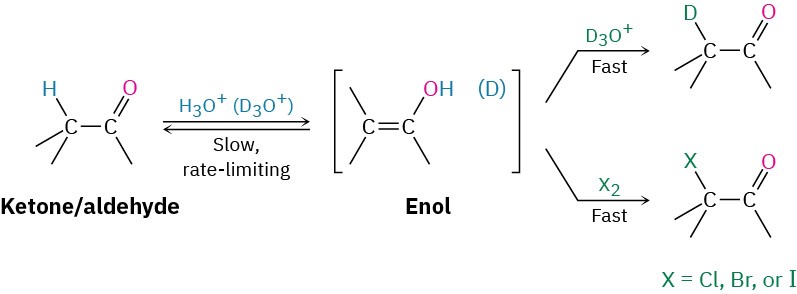
Evidence for the mechanism shown in Figure 22.4 includes the observation that acid- catalyzed halogenations show second-order kinetics and follow the rate law
Reaction rate = 𝑘[Ketone][H+]
In other words, the rate of halogenation depends only on the concentrations of ketone and acid and is independent of halogen concentration. Since halogen is not involved in the rate-limiting step, chlorination, bromination, and iodination of a given substrate all occur at the same rate.
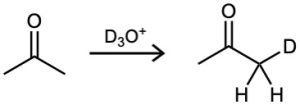
Furthermore, if an aldehyde or ketone is treated with D3O+, the acidic α hydrogens are replaced by deuterium. For a given ketone, the rate of deuterium exchange is identical to the rate of halogenation, implying that a common intermediate—presumably the enol—is involved in both processes.
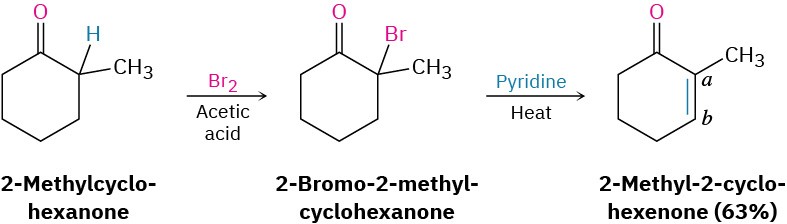
α-Bromo ketones are useful in the laboratory because they can be dehydrobrominated by base treatment to yield α,β-unsaturated ketones. For example, 2-methylcyclohexanone gives 2-bromo-2-methylcyclohexanone on halogenation, and the α-bromo ketone gives 2-methyl-2-cyclohexenone when heated in pyridine. The reaction takes place by an E2 elimination pathway (Section 11.8) and is a good method for introducing a C═C bond into a molecule. Note that bromination of 2-methylcyclohexanone occurs primarily on the more highly substituted α position because the more highly substituted enol is favored over the less highly substituted one (Section 7.6).
Problem 22-4
Write the complete mechanism for the deuteration of acetone on treatment with D3O+.
Problem 22-5
Show how you might prepare 1-penten-3-one from 3-pentanone.

