The many reactions that occur in the cells of living organisms are collectively called metabolism. The pathways that break down larger molecules into smaller ones are called catabolism, and the pathways that synthesize larger biomolecules from smaller ones are known as anabolism. Catabolic reaction pathways are usually exergonic and release energy, while anabolic pathways are often endergonic and absorb energy. Catabolism can be divided into the four stages shown in Figure 29.2.
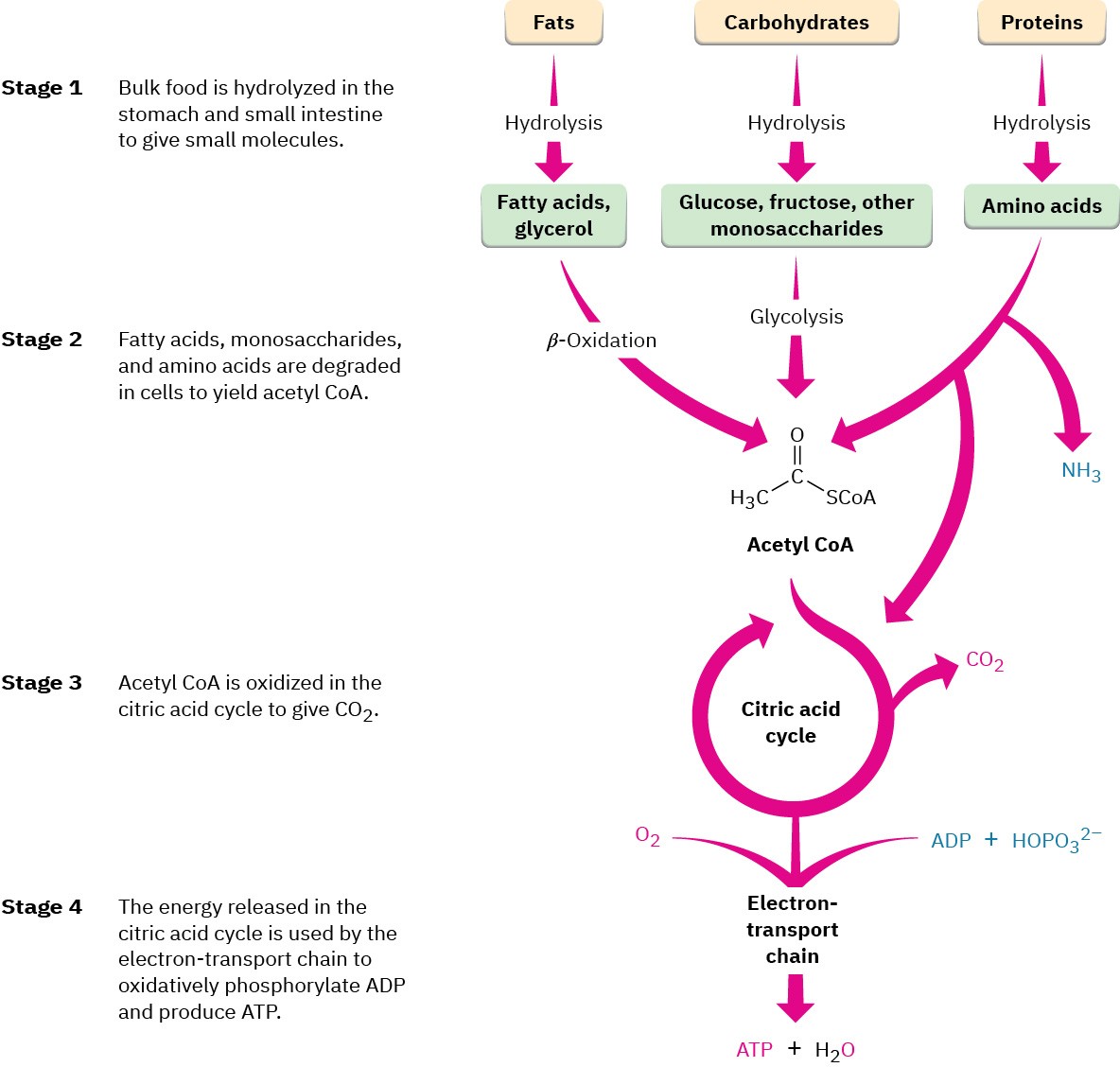
Figure 29.2 An overview of catabolic pathways for the degradation of food and the production of biochemical energy. The ultimate products of food catabolism are CO2 and H2O, with the energy released in the citric acid cycle used to drive the endergonic synthesis of adenosine triphosphate (ATP) from adenosine diphosphate (ADP) plus hydrogen phosphate ion, HOPO32–.
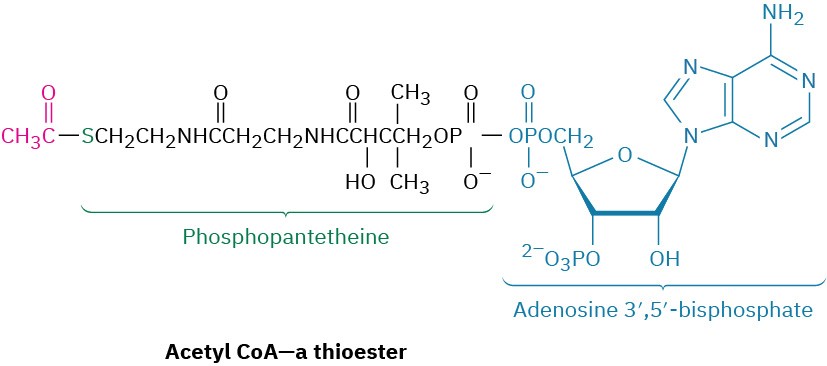
In the first catabolic stage, commonly called digestion, food is broken down in the mouth, stomach, and small intestine by hydrolysis of ester, acetal (glycoside), and amide (peptide) bonds to yield fatty acids, simple sugars, and amino acids. These smaller molecules are then absorbed and further degraded in the second stage of catabolism to yield acetyl groups attached by a thioester bond to the large carrier molecule, coenzyme A. The resultant compound, acetyl coenzyme A (acetyl CoA), is a key substance in the metabolism of food molecules and in many other biological pathways. As noted in Section 21.8, the acetyl group in acetyl CoA is linked to the sulfur atom of phosphopantetheine, which is itself linked to adenosine 3′,5′-bisphosphate.
Acetyl groups are oxidized inside cellular mitochondria in the third stage of catabolism, the citric acid cycle, to yield CO2. (We’ll see the details of the process in Section 29.7.) Like most oxidations, this stage releases a large amount of energy, which is used in the fourth stage, the electron-transport chain, to accomplish the endergonic phosphorylation of adenosine diphosphate (ADP) with hydrogen phosphate ion (HOPO32–, abbreviated Pi) to give adenosine triphosphate (ATP).
As the final result of food catabolism, ATP has been called the “energy currency” of the cell. Catabolic reactions “buy” ATP with the energy they release to synthesize it from ADP and hydrogen phosphate ion. Anabolic reactions then spend the ATP by transferring a phosphate group to another molecule, thereby regenerating ADP. Energy production and use in living organisms thus revolves around the ATP ⇄ ADP interconversion.
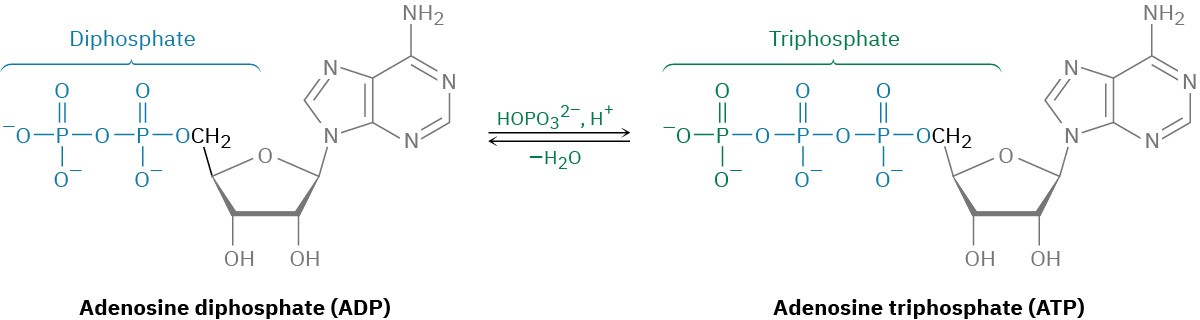
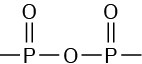
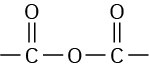 ADP and ATP are both phosphoric acid anhydrides, which containlinkages analogous to thelinkage in carboxylic acid anhydrides. Just as carboxylic acid
ADP and ATP are both phosphoric acid anhydrides, which containlinkages analogous to thelinkage in carboxylic acid anhydrides. Just as carboxylic acid
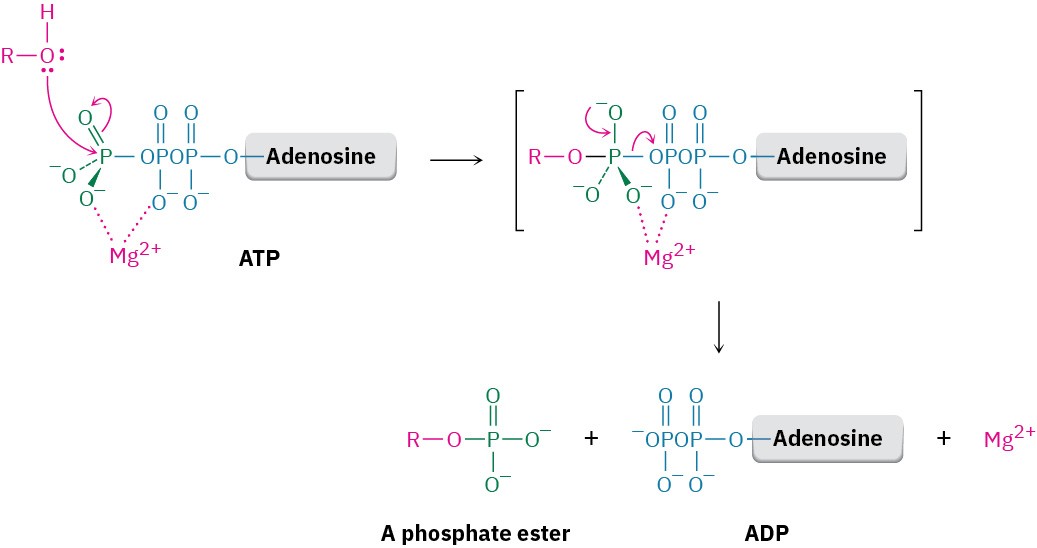
anhydrides react with alcohols by breaking a C–O bond and forming a carboxylic ester, ROCOR’ (Section 21.5), phosphoric acid anhydrides react with alcohols by breaking a P–O bond and forming a phosphate ester, ROPO32–. The reaction is, in effect, a nucleophilic acyl substitution at phosphorus. Note that phosphorylation reactions with ATP generally require the presence of a divalent metal cation in the enzyme, usually Mg2+, to form a Lewis acid–base complex with the phosphate oxygen atoms and to neutralize negative charge.
How does the body use ATP? Recall from Section 6.7 that the free-energy change ΔG must be negative and energy must be released for a reaction to be energetically favorable and occur spontaneously. If ΔG is positive, the reaction is energetically unfavorable and the process can’t occur spontaneously.
For an energetically unfavorable reaction to occur, it must be “coupled” to an energetically favorable reaction so that the overall free-energy change for the two reactions together is favorable. To understand what it means for reactions to be coupled, imagine that reaction 1 does not occur to any reasonable extent because it has a small equilibrium constant and is energetically unfavorable; that is, the reaction has ΔG > 0.
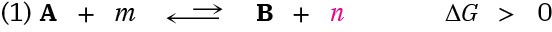
where A and B are the biochemically “important” substances while m and n are enzyme cofactors, H2O, or other small molecules.
Imagine also that product n can react with substance o to yield p and q in a second, highly favorable reaction that has a large equilibrium constant and ΔG << 0.
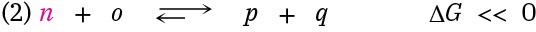
Taking the two reactions together, they share, or are coupled through, the common intermediate n, which is a product in the first reaction and a reactant in the second. When even a tiny amount of n is formed in reaction 1, it undergoes essentially complete conversion in reaction 2, thereby removing it from the first equilibrium and forcing reaction 1 to continually replenish n until reactant A is gone. That is, the two reactions
added together have a favorable ΔG < 0, and we say that the favorable reaction 2 “drives” the unfavorable reaction 1. Because the two reactions are coupled through n, the transformation of A to B becomes favorable.
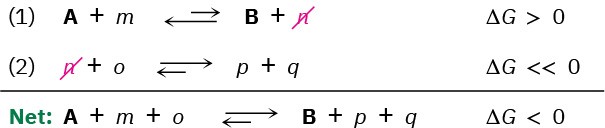
For an example of two reactions that are coupled, look at the phosphorylation reaction of glucose to yield glucose 6-phosphate plus water, an important step in the breakdown of dietary carbohydrates.
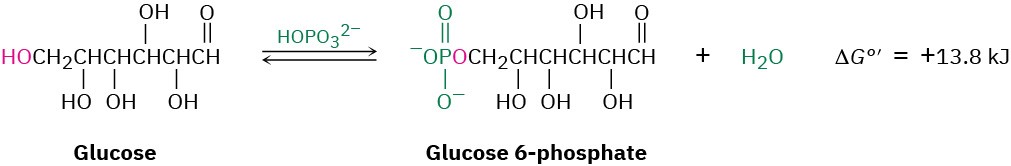
The reaction of glucose with HOPO32– does not occur spontaneously because it is energetically unfavorable, with ΔG°’ = 13.8 kJ/mol. (The standard free-energy change for a biological reaction is denoted ΔG°’ and refers to a process in which reactants and products have a concentration of 1.0 M in a solution with pH = 7.) At the same time, however, the reaction of water with ATP to yield ADP plus HOPO32– is strongly favorable, with ΔG°’ = –
30.5 kJ/mol. When the two reactions are coupled, glucose reacts with ATP to yield glucose 6-phosphate plus ADP in a reaction that is favorable by about 16.7 kJ/mol (4.0 kcal/mol). That is, ATP drives the phosphorylation reaction of glucose.
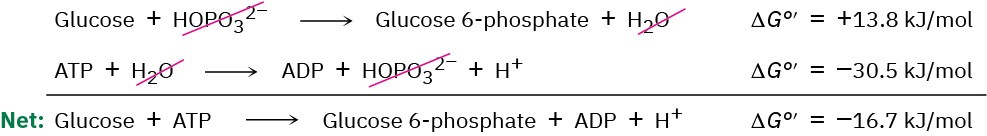
It’s this ability to drive otherwise unfavorable phosphorylation reactions that makes ATP so useful. The resultant phosphates are much more reactive as leaving groups in nucleophilic substitutions and eliminations than the alcohols they’re derived from and are therefore more chemically useful.
Problem 29-1
One of the steps in fat metabolism is the reaction of glycerol (1,2,3-propanetriol) with ATP to yield glycerol 1-phosphate. Write the reaction, and draw the structure of glycerol 1- phosphate.

