Look back once again at the definition of aromaticity in Section 15.3: a cyclic, conjugated molecule containing 4n + 2 π electrons. Nothing in this definition says that the atoms in the ring must be carbon. In fact, heterocyclic compounds can also be aromatic. A heterocycle is a cyclic compound that contains atoms of more than one element in its ring, usually carbon along with nitrogen, oxygen, or sulfur. Pyridine and pyrimidine, for example, are six-membered heterocycles with carbon and nitrogen in their rings (Figure 15.9).
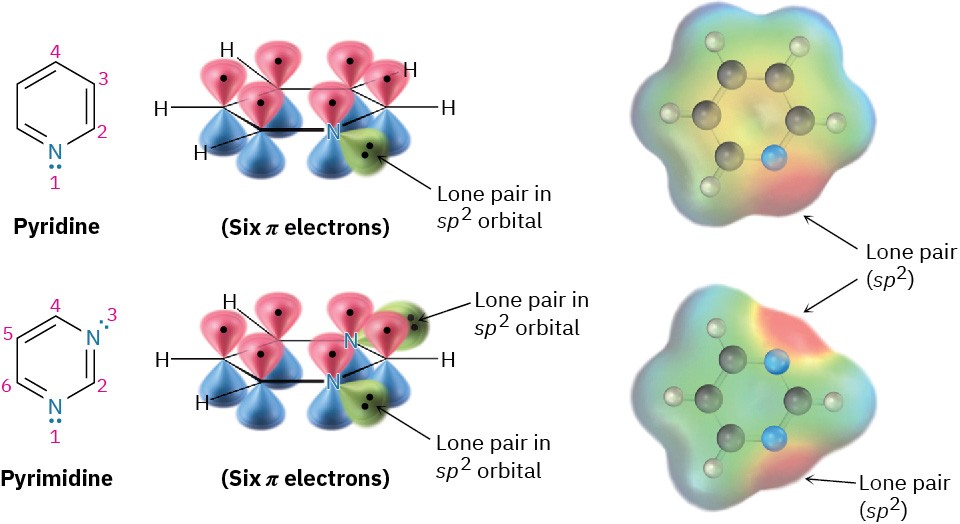
Figure 15.9 Pyridine and pyrimidine are nitrogen-containing aromatic heterocycles with π electron arrangements like that of benzene. Both have a lone pair of electrons on nitrogen in an sp2 orbital in the plane of the ring.
Pyridine is much like benzene in its π electron structure. Each of the five sp2-hybridized carbons has a p orbital perpendicular to the plane of the ring, and each p orbital contains one π electron. The nitrogen atom is also sp2-hybridized and has one electron in a p orbital, bringing the total to six π electrons. The nitrogen lone-pair electrons (red in an electrostatic potential map) are in a sp2 orbital in the plane of the ring and are not part of the aromatic π system. Pyrimidine, also shown in Figure 15.9), is a benzene analog that has two nitrogen atoms in a six-membered, unsaturated ring. Both nitrogens are sp2-hybridized, and each contributes one electron to the aromatic π system.
Pyrrole (spelled with two r’s and one l) and imidazole are five-membered heterocycles, yet both have six π electrons and are aromatic. In pyrrole, each of the four sp2-hybridized carbons contributes one π electron and the sp2-hybridized nitrogen atom contributes the two from its lone pair, which occupies a p orbital (Figure 15.10). Imidazole, also shown in (Figure 15.10, is an analog of pyrrole that has two nitrogen atoms in a five-membered, unsaturated ring. Both nitrogens are sp2-hybridized, but one is in a double bond and contributes only one electron to the aromatic π system whereas the other is not in a double bond and contributes two from its lone pair.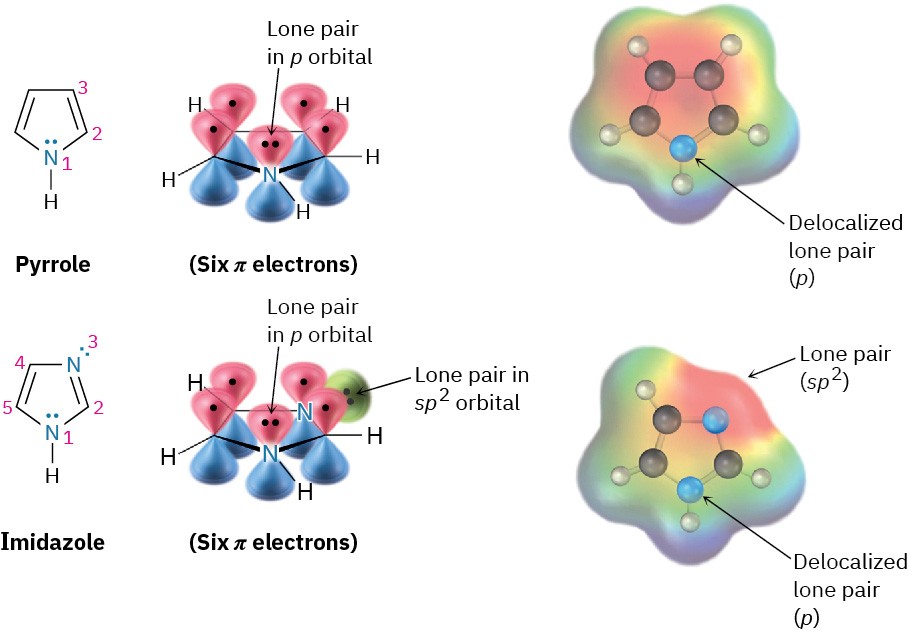
Figure 15.10 Pyrrole and imidazole are five-membered, nitrogen-containing heterocycles but have six-π-electron arrangements like that of the cyclopentadienyl anion. Both have a lone pair of electrons on nitrogen in a p orbital perpendicular to the ring.
Note that nitrogen atoms have different roles depending on the structure of the molecule. The nitrogen atoms in pyridine and pyrimidine are both in double bonds and contribute only one π electron to the aromatic sextet, just as a carbon atom in benzene does. The nitrogen atom in pyrrole, however, is not in a double bond and contributes two π electrons (its lone pair) to the aromatic sextet. In imidazole, both kinds of nitrogen are present in the same molecule—a double-bonded “pyridine-like” nitrogen that contributes one π electron and a “pyrrole-like” nitrogen that contributes two.
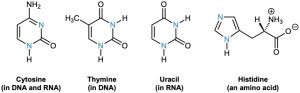
Pyrimidine and imidazole rings are particularly important in biological chemistry. Pyrimidine, for instance, is the parent ring system in cytosine, thymine, and uracil, three of the five heterocyclic amine bases found in nucleic acids. An aromatic imidazole ring is present in histidine, one of the 20 amino acids found in proteins.
Worked Example 15.1
Accounting for the Aromaticity of a HeterocycleThiophene, a sulfur-containing heterocycle, undergoes typical aromatic substitution reactions rather than addition reactions. Why is thiophene aromatic?

Strategy
Recall the requirements for aromaticity—a planar, cyclic, conjugated molecule with 4n + 2π electrons—and see how these requirements apply to thiophene.SolutionThiophene is the sulfur analog of pyrrole. The sulfur atom is sp2-hybridized and has a lone pair of electrons in a p orbital perpendicular to the plane of the ring. Sulfur also has a second lone pair of electrons in the ring plane.
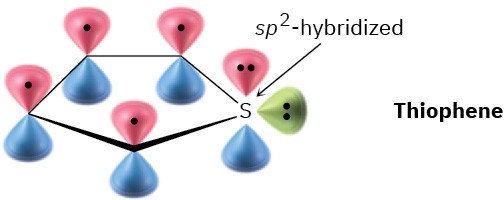
Problem 15-9
Draw an orbital picture of furan to show how the molecule is aromatic.
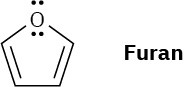
Problem 15-10
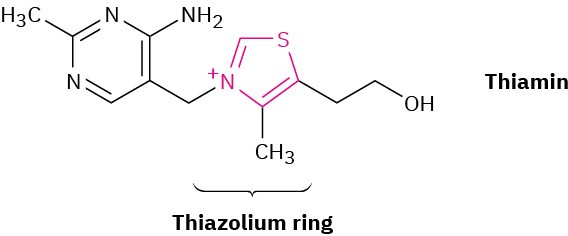
Thiamin, or vitamin B1, contains a positively charged five-membered nitrogen–sulfur heterocycle called a thiazolium ring. Explain why the thiazolium ring is aromatic.