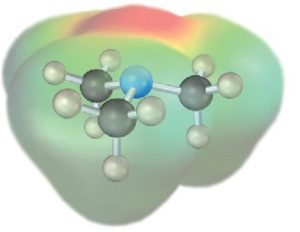
The chemistry of amines is dominated by the lone pair of electrons on nitrogen, which makes amines both basic and nucleophilic. They react with acids to form acid–base salts, and they react with electrophiles in many of the polar reactions seen in past chapters. Note in the following electrostatic potential map of trimethylamine how the negative (red) region corresponds to the lone pair of electrons on nitrogen.
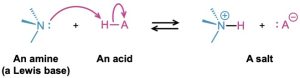
Amines are much stronger bases than alcohols and ethers, their oxygen-containing analogs. When an amine is dissolved in water, an equilibrium is established in which water acts as an acid and transfers a proton to the amine. Just as the acid strength of a carboxylic acid can be measured by defining an acidity constant Ka (Section 2.8), the base strength of an amine can be measured by defining an analogous basicity constant Kb. The larger the value of Kb and the smaller the value of pKb, the more favorable the proton-transfer equilibrium and the stronger the base.
For the reaction
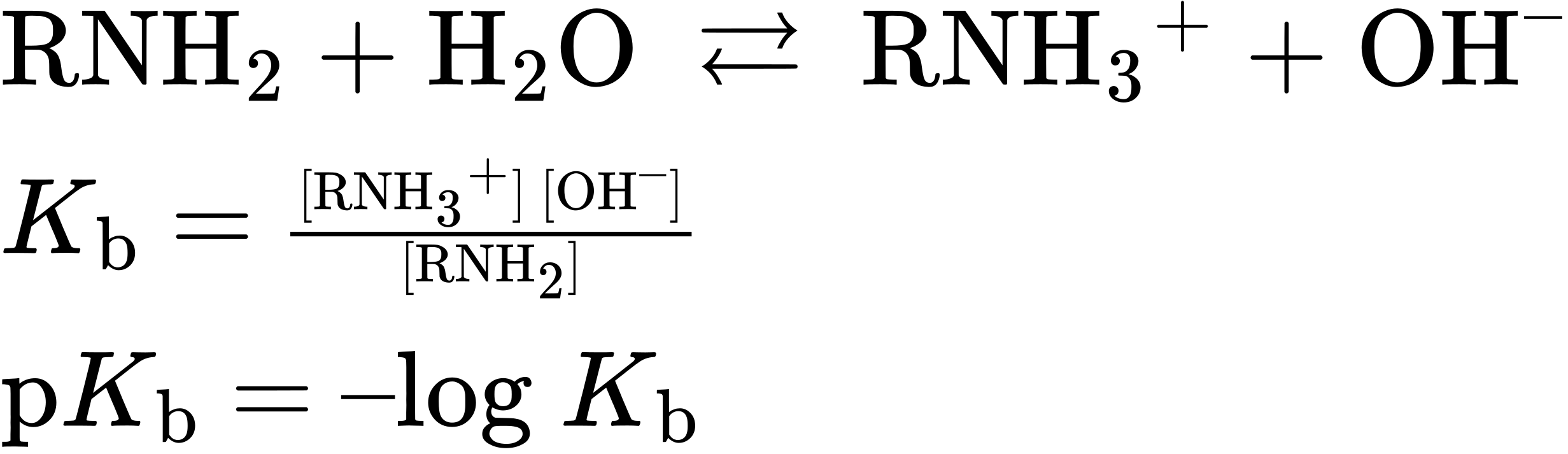
In practice, Kb values are not often used. Instead, the most convenient way to measure the basicity of an amine (RNH2) is to look at the acidity of the corresponding ammonium ion (RNH3+).
For the reaction
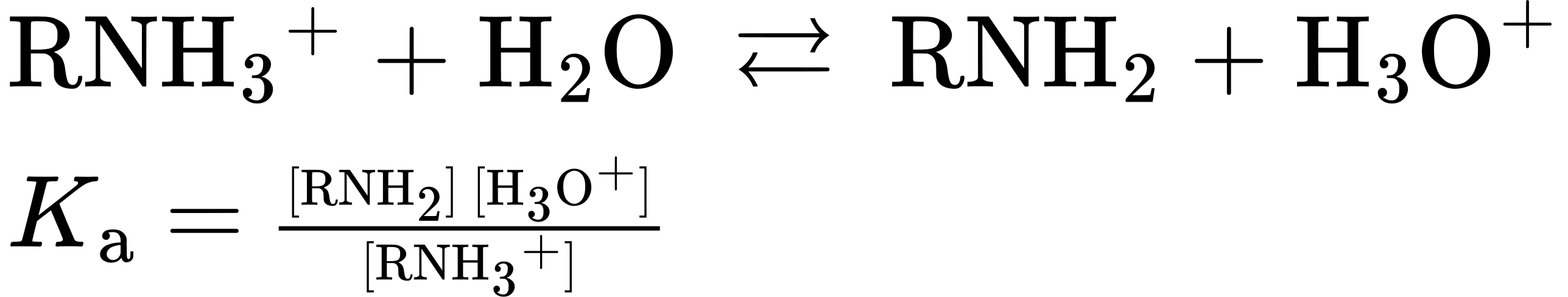
so
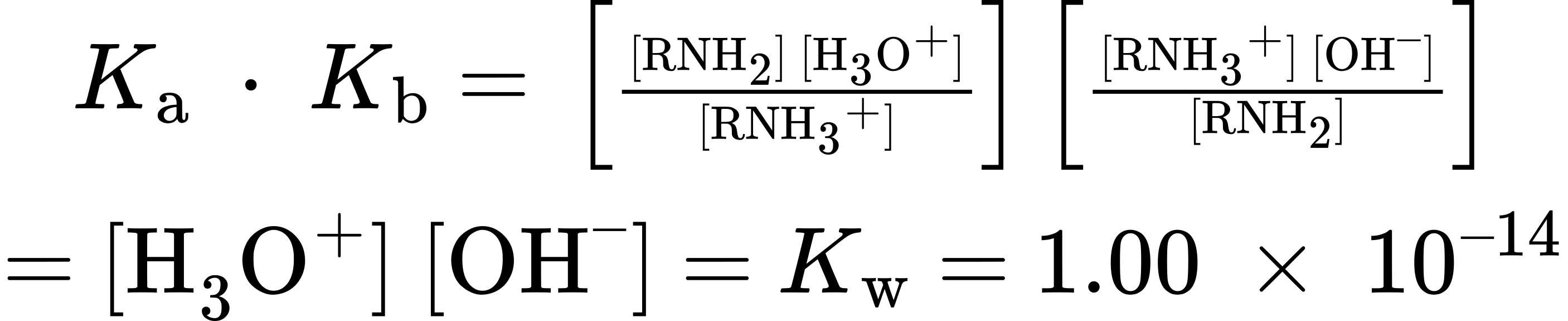
Thus
[latex]K_a = \frac{K_w}{K_b}[/latex] and
[latex]K_b = \frac{K_w}{K_a}[/latex] and
[latex]pK_a + pK_b = 14[/latex]
These equations say that the Kb of an amine multiplied by the Ka of the corresponding ammonium ion is equal to Kw, the ion-product constant for water (1.00 × 10–14). Thus, if we know Ka for an ammonium ion, we also know Kb for the corresponding amine base because Kb = Kw/Ka. The more acidic the ammonium ion, the less tightly the proton is held and the weaker the corresponding base. That is, a weaker base has an ammonium ion with a smaller pKa and a stronger base has an ammonium ion with a larger pKa.
|
Weaker base |
Smaller pKa for ammonium ion |
|
Stronger base |
Larger pKa for ammonium ion |
Table 24.1 lists pKa values of the ammonium ions from a variety of amines and indicates that there is a substantial range of amine basicities. Most simple alkylamines are similar in their base strength, with pKa’s for their ammonium ions in the narrow range 10 to 11.
Arylamines, however, are considerably less basic than alkylamines, as are the heterocyclic amines pyridine and pyrrole.
Table 24.1 Basicity of Some Common Amines
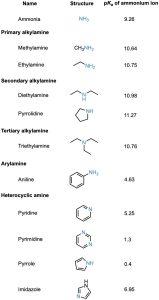
In contrast with amines, amides (RCONH2) are nonbasic. Amides aren’t protonated by aqueous acids, and they are poor nucleophiles. The main reason for this difference in basicity between amines and amides is that an amide is stabilized by delocalization of the nitrogen lone-pair electrons through orbital overlap with the carbonyl group. In resonance terms, amides are more stable and less reactive than amines because they are hybrids of two resonance forms. This amide resonance stabilization is lost when the nitrogen atom is protonated, so protonation is disfavored. The following electrostatic potential maps clearly show a reduced electron density on the amide nitrogen.
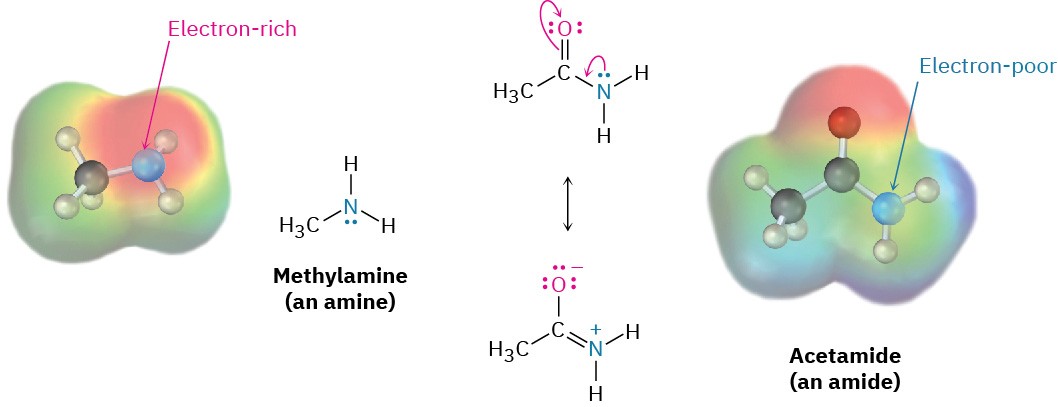
To purify amines, it’s often possible to take advantage of their basicity. For example, if a mixture of a basic amine and a neutral compound such as a ketone or alcohol is dissolved in an organic solvent and aqueous acid is added, the basic amine dissolves in the water layer as its protonated salt, while the neutral compound remains in the organic solvent layer.
Separation of the water layer and neutralization of the ammonium ion by addition of NaOH then provides the pure amine (Figure 24.4)
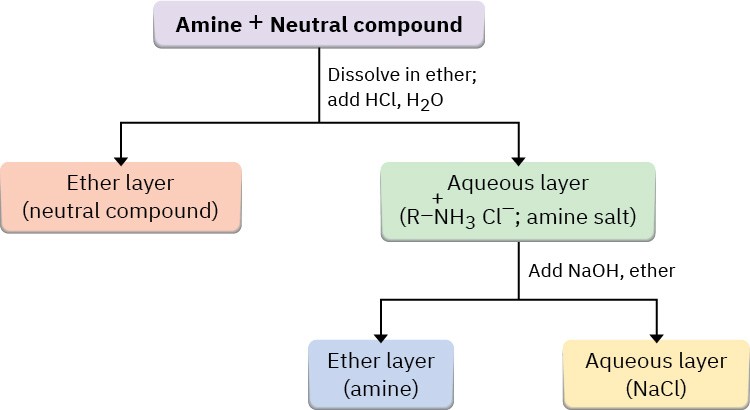
Figure 24.4 Separation and purification of an amine component from a mixture by extraction of its ammonium salt into water.
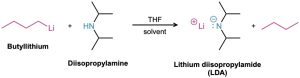
In addition to their behavior as bases, primary and secondary amines can also act as very weak acids because an N–H proton can be removed by a sufficiently strong base. We’ve seen, for example, how diisopropylamine (pKa ≈ 36) reacts with butyllithium to yield lithium diisopropylamide (LDA; Section 22.5). Dialkylamine anions like LDA are very strong bases that are often used in laboratory organic chemistry for the generation of enolate ions from carbonyl compounds (Section 22.7). They are not, however, encountered in biological chemistry.
Problem 24-4
Which compound in each of the following pairs is more basic?
(a) CH3CH2NH2 or CH3CH2CONH2
(b) NaOH or CH3NH2
(c) CH3NHCH3 or pyridine
Problem 24-5
The benzylammonium ion (C6H5CH2NH3+) has pKa = 9.33, and the propylammonium ion has pKa = 10.71. Which is the stronger base, benzylamine or propylamine? What are the pKb’s of benzylamine and propylamine?

