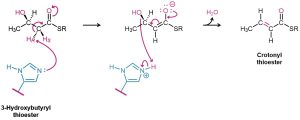
All three elimination reactions—E2, E1, and E1cB—occur in biological pathways, but the E1cB mechanism is particularly common. The substrate usually contains an alcohol leaving group rather than an alkyl halide, and the H atom removed is usually adjacent to a carbonyl group. Thus, 3-hydroxy carbonyl compounds are frequently converted to unsaturated carbonyl compounds by elimination reactions. A typical example occurs during the biosynthesis of fats and oils when a 3-hydroxybutyryl thioester is dehydrated to the corresponding unsaturated (crotonyl) thioester. The base in this reaction is a histidine amino acid in the enzyme, and the loss of the –OH group is assisted by simultaneous protonation.