Glucose is the body’s primary fuel when food is plentiful, but in times of fasting or prolonged exercise, glucose stores can become depleted. Most tissues then begin metabolizing fats as their source of acetyl CoA, but the brain is different. The brain relies almost entirely on glucose for fuel and is dependent on receiving a continuous supply in the blood. When the supply of glucose fails, even for a brief time, irreversible damage can occur. Thus, a pathway for synthesizing glucose from simple precursors is crucial.
Higher organisms are not able to synthesize glucose from acetyl CoA but must instead use one of the three-carbon precursors lactate, glycerol, or alanine, all of which are readily converted into pyruvate.
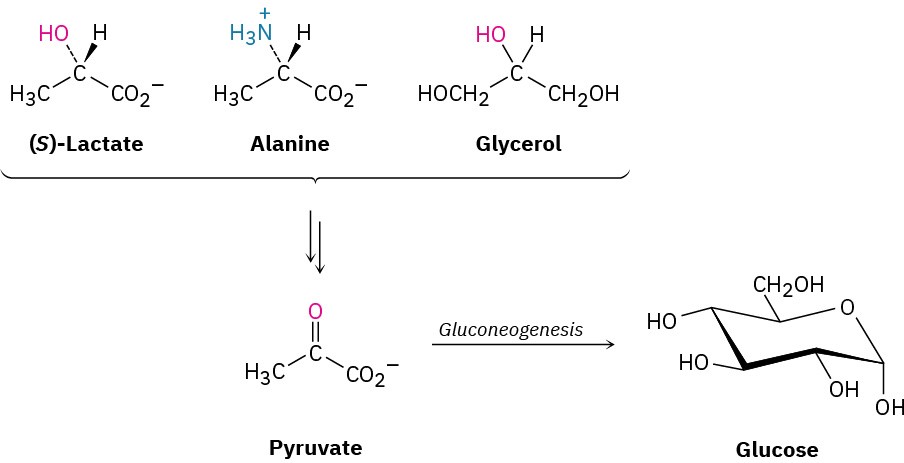
Pyruvate then becomes the starting point for gluconeogenesis, the 11-step biosynthetic pathway by which organisms make glucose (Figure 29.15). The gluconeogenesis pathway is not the reverse of the glycolysis pathway by which glucose is degraded. As with the catabolic and anabolic pathways for fatty acids (Section 29.3 and Section 29.4), the catabolic and anabolic pathways for carbohydrates differ in some details so that both are energetically favorable.
Figure 29.15 MECHANISM
The gluconeogenesis pathway for the biosynthesis of glucose from pyruvate.
Individual steps are explained in the text.
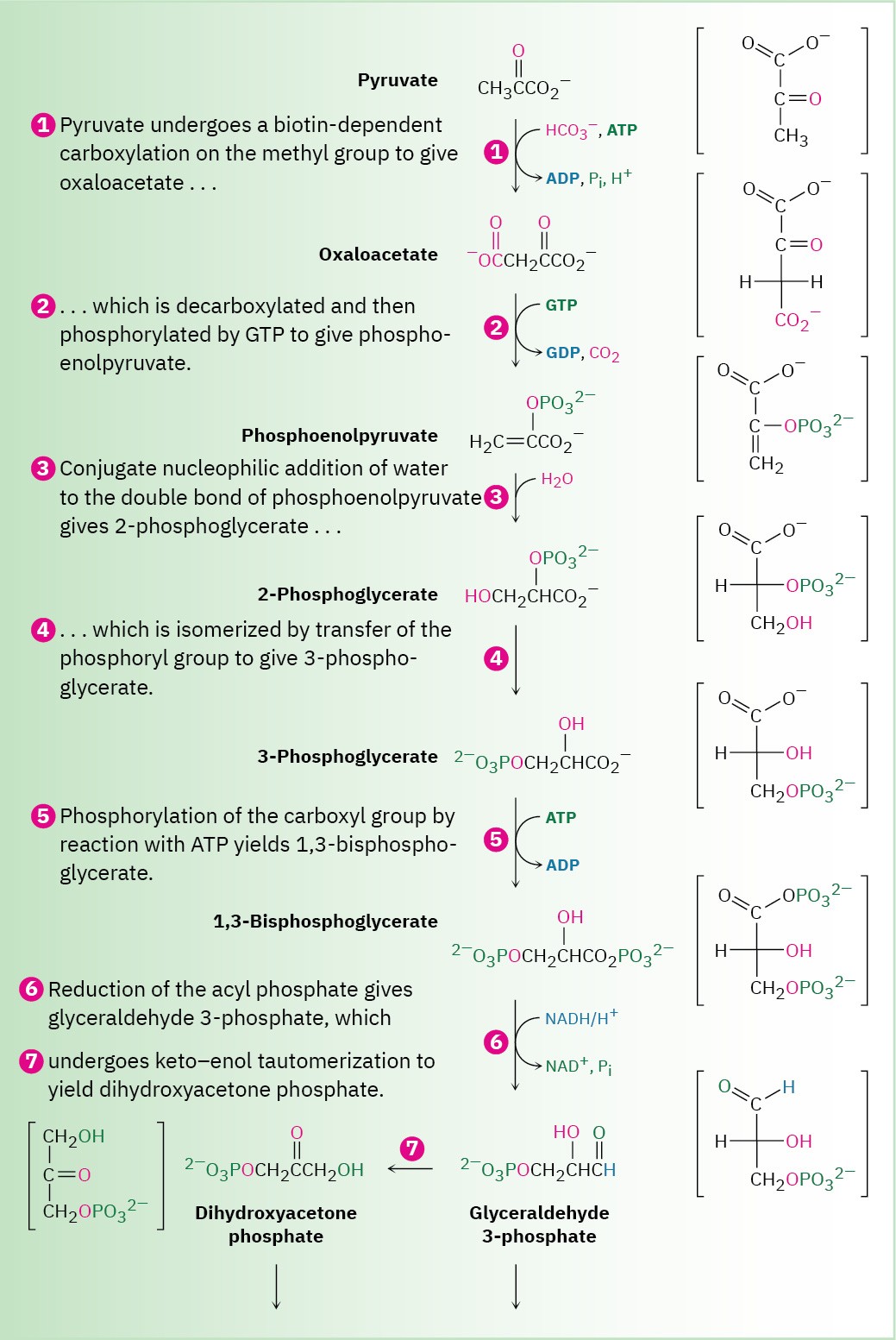
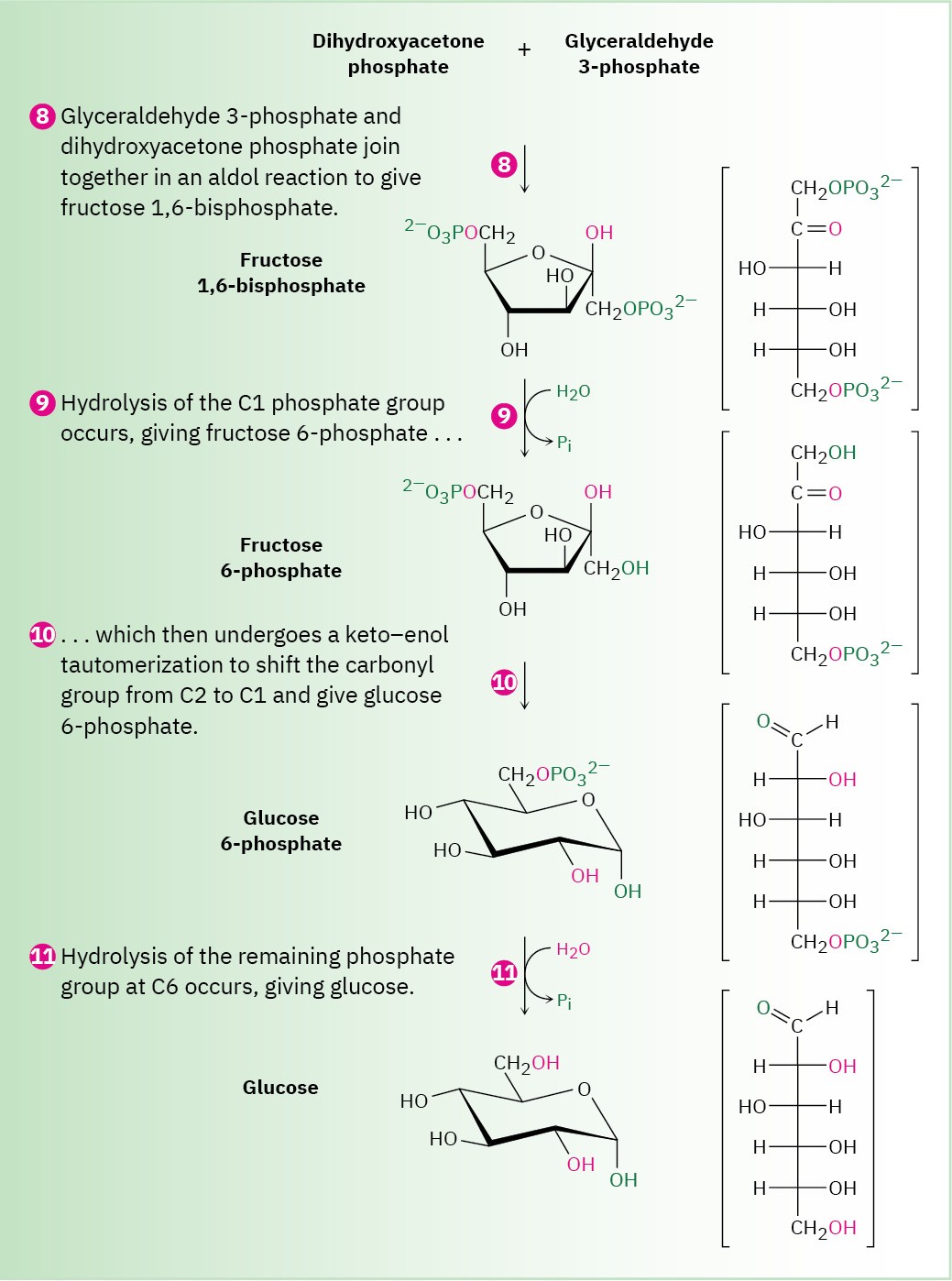
Figure 29.16 MECHANISM
(Continued)
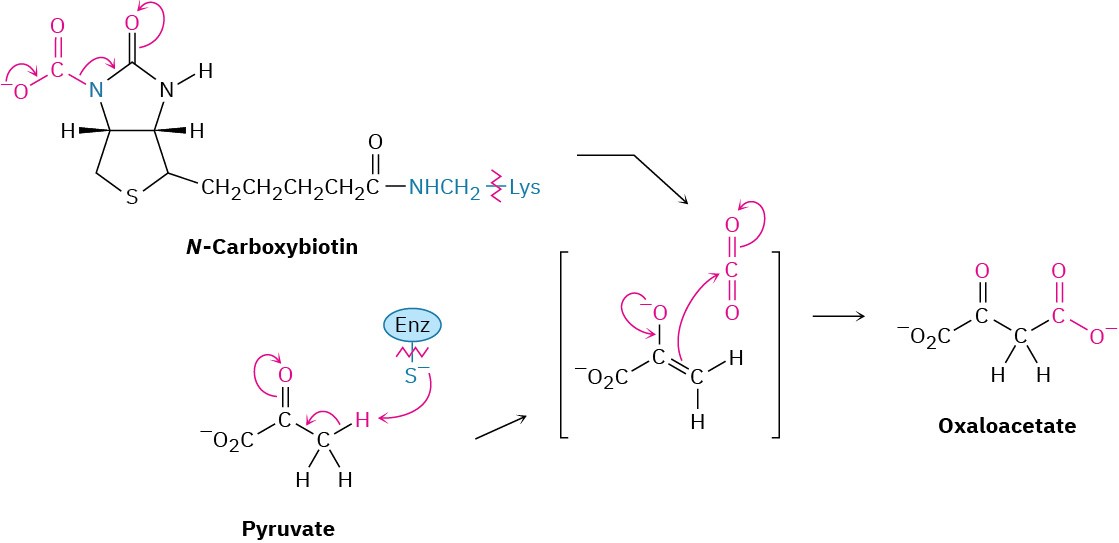
Step 1 of Figure 29.15: Carboxylation
Gluconeogenesis begins with the carboxylation of pyruvate to yield oxaloacetate. The reaction is catalyzed by pyruvate carboxylase and requires ATP, bicarbonate ion, and the coenzyme biotin, which acts as a carrier to transport CO2 to the enzyme active site. The mechanism is analogous to that of step 3 in fatty-acid biosynthesis (Figure 29.6), in which acetyl CoA is carboxylated to yield malonyl CoA.
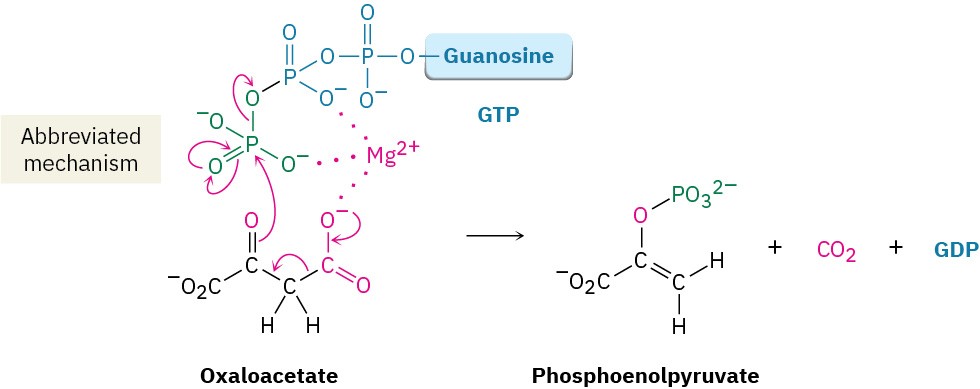
Step 2 of Figure 29.15: Decarboxylation and Phosphorylation
Decarboxylation of oxaloacetate, a β-keto acid, occurs by the typical retro-aldol mechanism like that in step 3 in the citric acid cycle (Figure 29.14), and phosphorylation of the resultant pyruvate enolate ion by GTP occurs concurrently to give phosphoenolpyruvate.
This reaction is catalyzed by phosphoenolpyruvate carboxykinase.
Steps 3–4 of Figure 29.15: Hydration and Isomerization
Conjugate nucleophilic addition of water to the double bond of phosphoenolpyruvate gives 2-phosphoglycerate by a process similar to that of step 7 in the citric acid cycle.
Phosphorylation of C3 and dephosphorylation of C2 then yields 3-phosphoglycerate. Mechanistically, these steps are the reverse of steps 9 and 8 in glycolysis (Figure 29.8), which have equilibrium constants near 1 so that substantial amounts of reactant and product are both present.
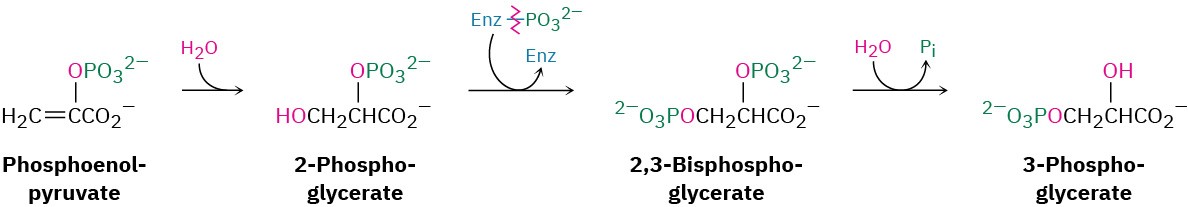
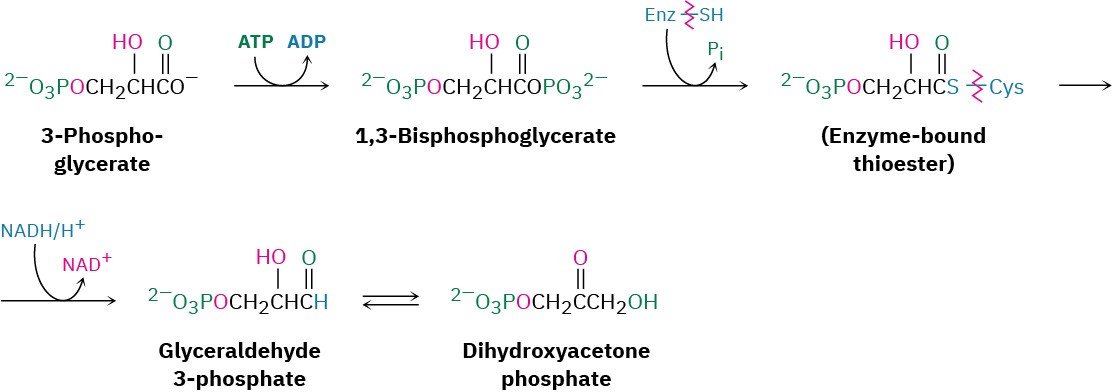
Steps 5–7 of Figure 29.15: Phosphorylation, Reduction, and Tautomerization
Reaction of 3-phosphoglycerate with ATP generates the corresponding acyl phosphate, 1,3- bisphosphoglycerate, which binds to the glyceraldehyde 3-phosphate dehydrogenase by a thioester bond to a cysteine residue. Reduction of the thioester by NADH/H+ yields the corresponding aldehyde, and keto–enol tautomerization of the aldehyde gives dihydroxyacetone phosphate. All three steps comprise a mechanistic reversal of the corresponding steps 7, 6, and 5 of glycolysis and have equilibrium constants near 1.
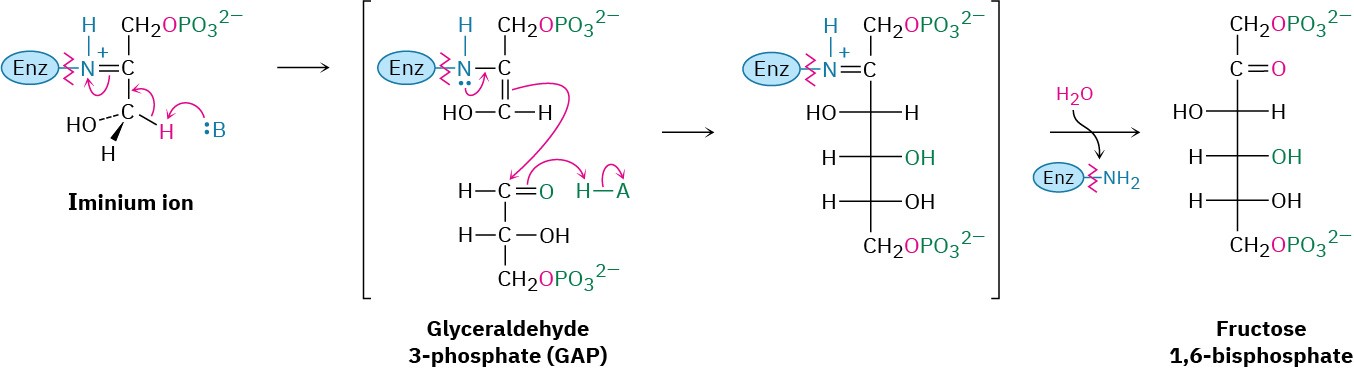
Step 8 of Figure 29.16: Aldol Reaction
Dihydroxyacetone phosphate and glyceraldehyde 3-phosphate, the two 3-carbon units produced in step 7, join by an aldol reaction to give fructose 1,6-bisphosphate, the reverse of step 4 in glycolysis (Figure 29.11). As in glycolysis, the reaction is catalyzed in plants and animals by a class I aldolase and takes place on an iminium ion formed by reaction of dihydroxyacetone phosphate with a side-chain lysine –NH2 group on the enzyme. Loss of a proton from the neighboring carbon then generates an enamine, an aldol-like reaction ensues, and the product is hydrolyzed.
Steps 9–10 of Figure 29.16: Hydrolysis and Isomerization
Hydrolysis of the phosphate group at C1 of fructose 1,6-bisphosphate gives fructose 6- phosphate. Although the result of the reaction is the exact opposite of step 3 in glycolysis, the mechanism is not. In glycolysis, phosphorylation is accomplished by reaction of fructose with ATP, with formation of ADP as by-product. The reverse of that process,
however—the reaction of fructose 1,6-bisphosphate with ADP to give fructose 6-phosphate and ATP—is energetically unfavorable because ATP is too high in energy. Thus, an alternative pathway is used in which the C1 phosphate group is removed by a direct hydrolysis reaction, catalyzed by fructose 1,6-bisphosphatase.
Following hydrolysis, keto–enol tautomerization of the carbonyl group from C2 to C1 gives glucose 6-phosphate. The isomerization is the reverse of step 2 in glycolysis.
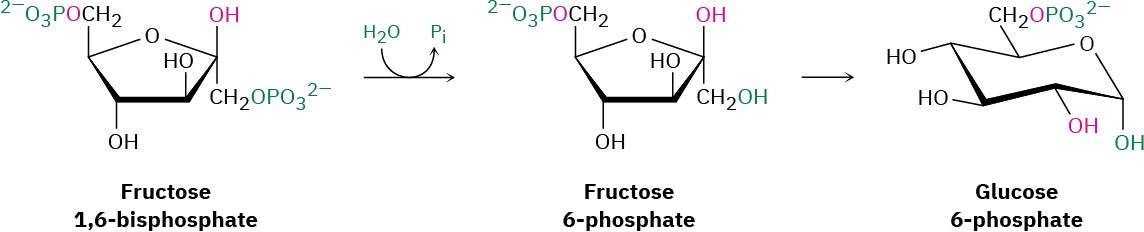
Step 11 of Figure 29.16: Hydrolysis
The final step in gluconeogenesis is the conversion of glucose 6-phosphate to glucose by a second phosphatase-catalyzed hydrolysis reaction. As just discussed for the hydrolysis of fructose 1,6-bisphosphate in step 9, and for the same energetic reasons, the mechanism of the glucose 6-phosphate hydrolysis is not the exact opposite of the corresponding step 1 in glycolysis.
Interestingly, however, the mechanisms of the two phosphate hydrolysis reactions in steps 9 and 11 are not the same. In step 9, water is the nucleophile, but in the glucose 6- phosphate reaction of step 11, a histidine residue on the enzyme attacks phosphorus, giving a phosphoryl enzyme intermediate that subsequently reacts with water.
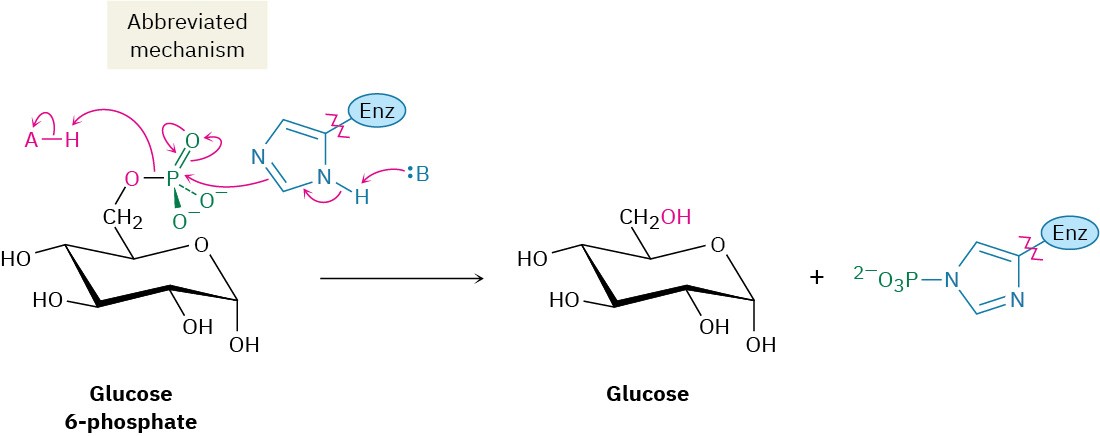
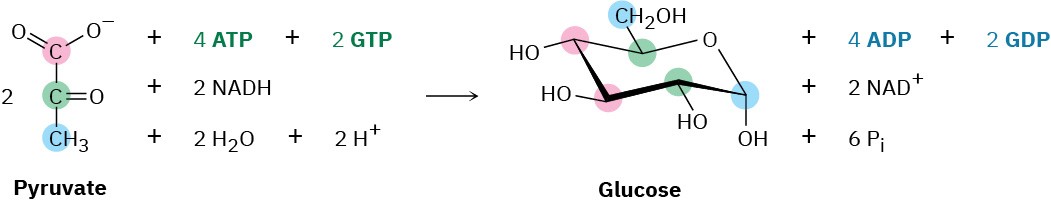
The overall result of gluconeogenesis is summarized by the following equation:
Problem 29-13
Write a mechanism for step 6 of gluconeogenesis, the reduction of 3-phosphoglyceryl phosphate with NADH/H+ to yield glyceraldehyde 3-phosphate.

