The fatty acids that result from triacylglycerol hydrolysis are converted into thioesters with coenzyme A and then catabolized by a repetitive four-step sequence of reactions called the β-oxidation pathway, shown in Figure 29.4. Each passage along the pathway results in the cleavage of an acetyl group from the end of the fatty-acid chain, until the entire molecule is ultimately degraded. As each acetyl group is produced, it enters the citric acid cycle and is further degraded to CO2, as we’ll see in Section 29.7.
Figure 29.4 MECHANISM
The four steps of the β-oxidation pathway, resulting in the cleavage of an acetyl group from the end of the fatty-acid chain. The key chain-shortening step is a retro- Claisen reaction of a β-keto thioester. Individual steps are explained in the text.
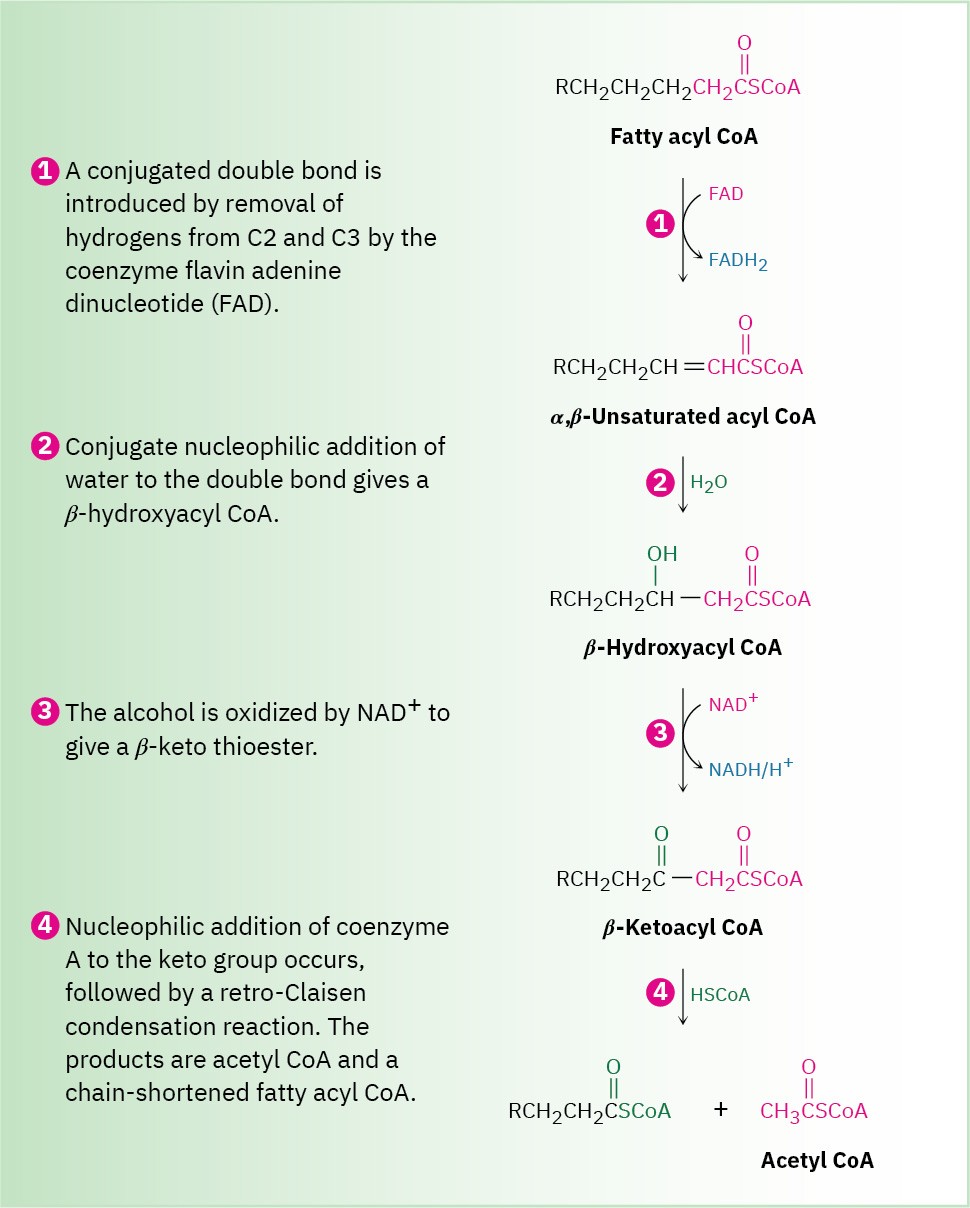
Step 1 of Figure 29.4: Introduction of a Double Bond
The β-oxidation pathway begins when two hydrogen atoms are removed from C2 and C3 of the fatty acyl CoA by one of a family of acyl-CoA dehydrogenases to yield an α,β– unsaturated acyl CoA. This kind of oxidation—the introduction of a conjugated double bond into a carbonyl compound—occurs frequently in biochemical pathways and usually involves the coenzyme flavin adenine dinucleotide (FAD). Reduced FADH2 is the by- product.
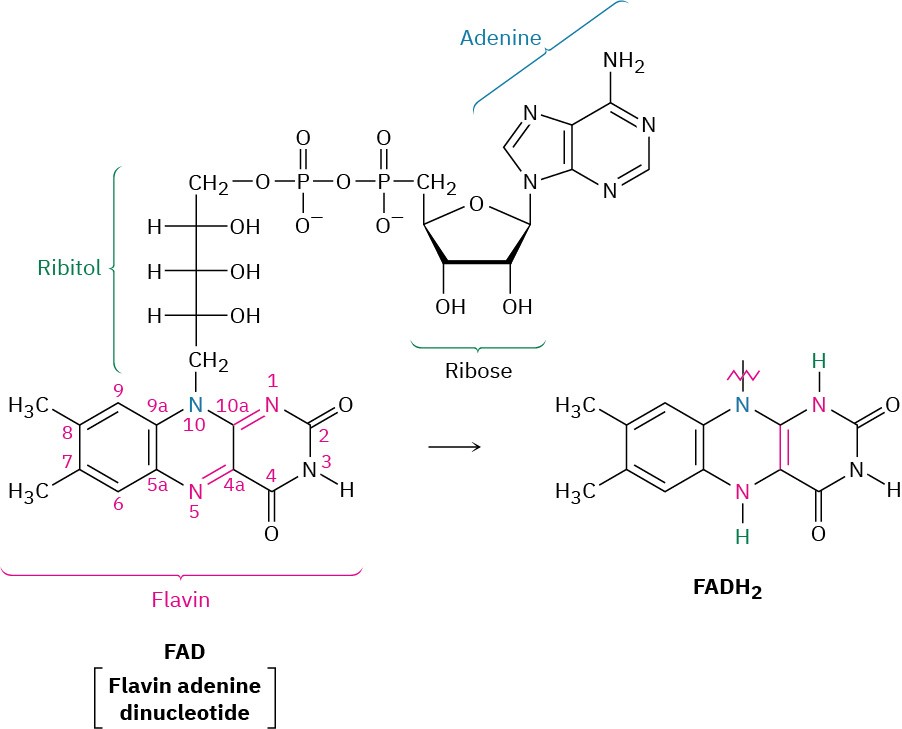
The mechanisms of FAD-catalyzed reactions are often difficult to establish because flavin coenzymes can follow both two-electron (polar) and one-electron (radical) pathways. As a result, extensive studies of the family of acyl-CoA dehydrogenases have not yet provided a clear picture of how these enzymes function. What is known is that: (1) The first step is abstraction of the pro-R hydrogen from the acidic α position of the acyl CoA to give a thioester enolate ion. Hydrogen-bonding between the acyl carbonyl group and the ribitol hydroxyls of FAD increases the acidity of the acyl group. (2) The pro-R hydrogen at the β position is transferred to FAD. (3) The α,β-unsaturated acyl CoA that results has a trans double bond.
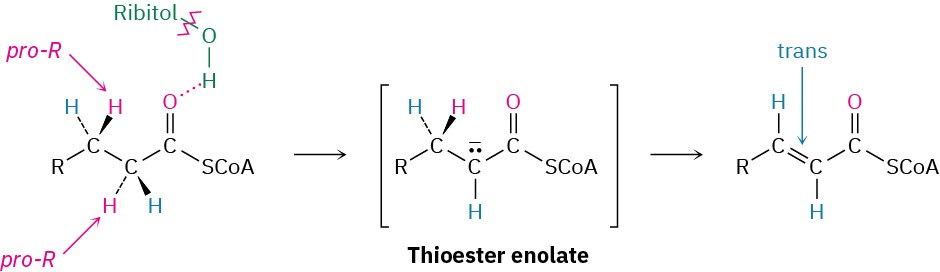
One suggested mechanism has the reaction taking place by a conjugate nucleophilic addition of hydride, analogous to what occurs during alcohol oxidations with NAD+. Electrons on the enolate ion might expel a β hydride ion, which could add to the doubly bonded N5 nitrogen on FAD. Protonation of the intermediate at N1 would give the product.
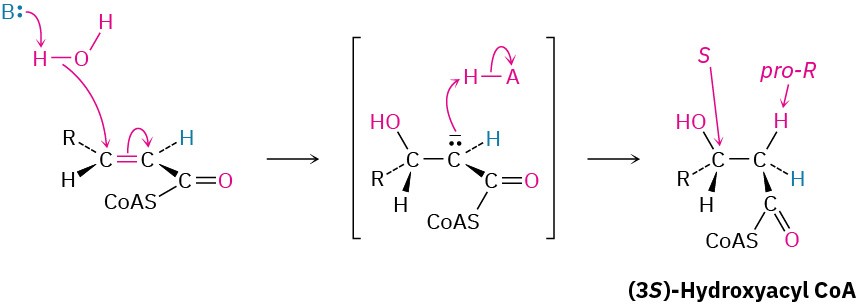
Step 2 of Figure 29.4: Conjugate Addition of Water
The α,β-unsaturated acyl CoA produced in step 1 reacts with water by a conjugate addition pathway (Section 19.13) to yield a β-hydroxyacyl CoA in a process catalyzed by enoyl CoA hydratase. Water as nucleophile adds to the β carbon of the double bond, yielding an intermediate thioester enolate ion that is protonated on the α position.
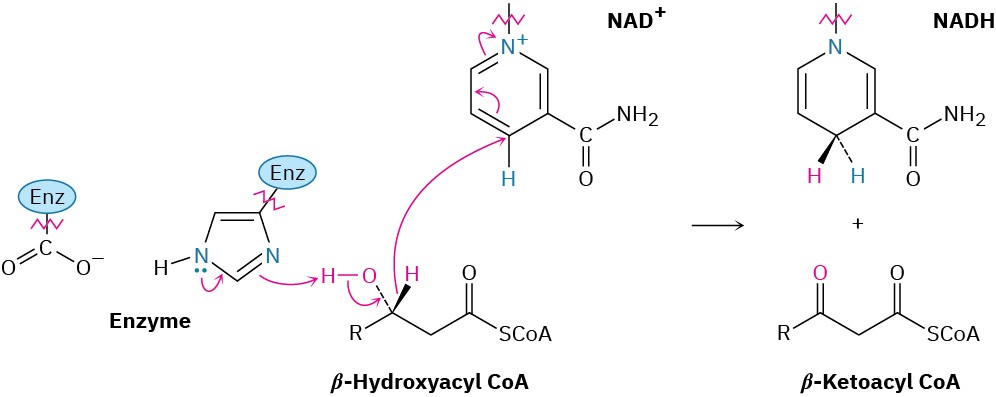
Step 3 of Figure 29.4: Alcohol Oxidation
The β-hydroxyacyl CoA from step 2 is oxidized to a β-ketoacyl CoA in a reaction catalyzed by one of a family of L-3-hydroxyacyl-CoA dehydrogenases, which differ in substrate specificity according to the chain length of the acyl group. As in the oxidation of sn-glycerol 3-phosphate to dihydroxyacetone phosphate mentioned at the end of Section 29.2, this alcohol oxidation requires NAD+ as a coenzyme and yields reduced NADH/H+ as by- product. Deprotonation of the hydroxyl group is carried out by a histidine residue at the active site.
Step 4 of Figure 29.4: Chain Cleavage
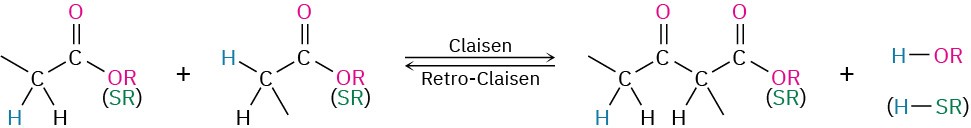
Acetyl CoA is split off from the chain in the final step of β-oxidation, leaving an acyl CoA that is two carbon atoms shorter than the original. The reaction is catalyzed by β-ketoacyl-CoA thiolase and is mechanistically the reverse of a Claisen condensation reaction (Section 23.7). In the forward direction, a Claisen condensation joins two esters together to form a
β-keto ester product. In the reverse direction, a retro-Claisen reaction splits apart a β-keto ester (or β-keto thioester in this case) to form two esters (or two thioesters).
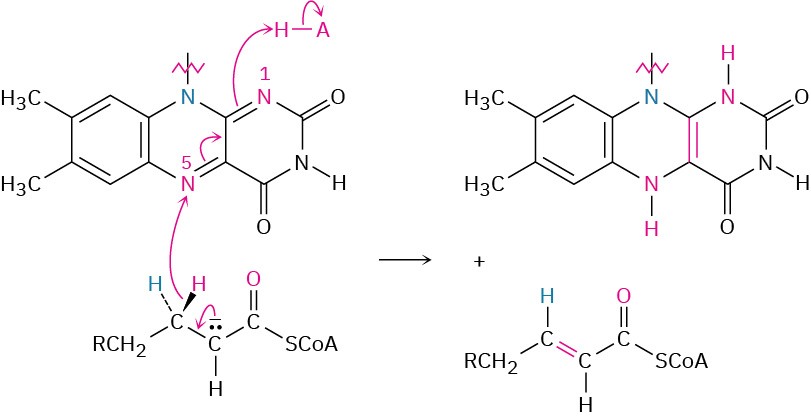
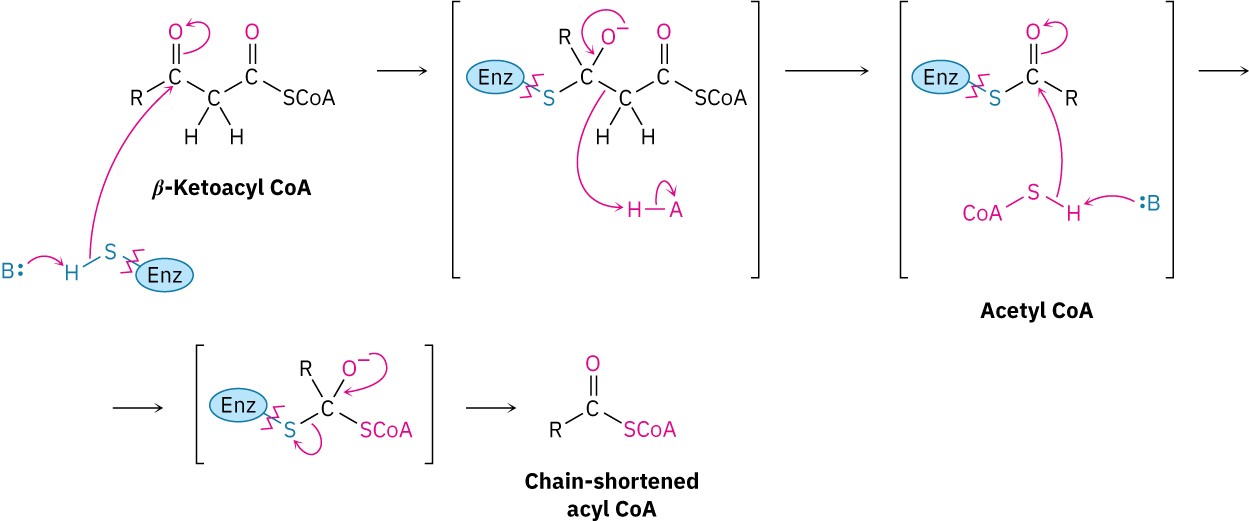
The retro-Claisen reaction occurs by nucleophilic addition of a cysteine −SH group on the enzyme to the keto group of the β-ketoacyl CoA to yield an alkoxide ion intermediate.
Cleavage of the C2–C3 bond then follows, with the expulsion of an acetyl CoA enolate ion that is immediately protonated. The enzyme-bound acyl group then undergoes nucleophilic acyl substitution by reaction with a molecule of coenzyme A, and the chain-shortened acyl CoA that results re-enters the β-oxidation pathway for further degradation.
Look at the catabolism of myristic acid shown in Figure 29.5 to see the overall results of the β-oxidation pathway. The first passage converts the 14-carbon myristoyl CoA into the 12- carbon lauroyl CoA plus acetyl CoA, the second passage converts lauroyl CoA into the 10- carbon caproyl CoA plus acetyl CoA, the third passage converts caproyl CoA into the 8- carbon capryloyl CoA, and so on. Note that the final passage produces two molecules of acetyl CoA because the precursor has four carbons.
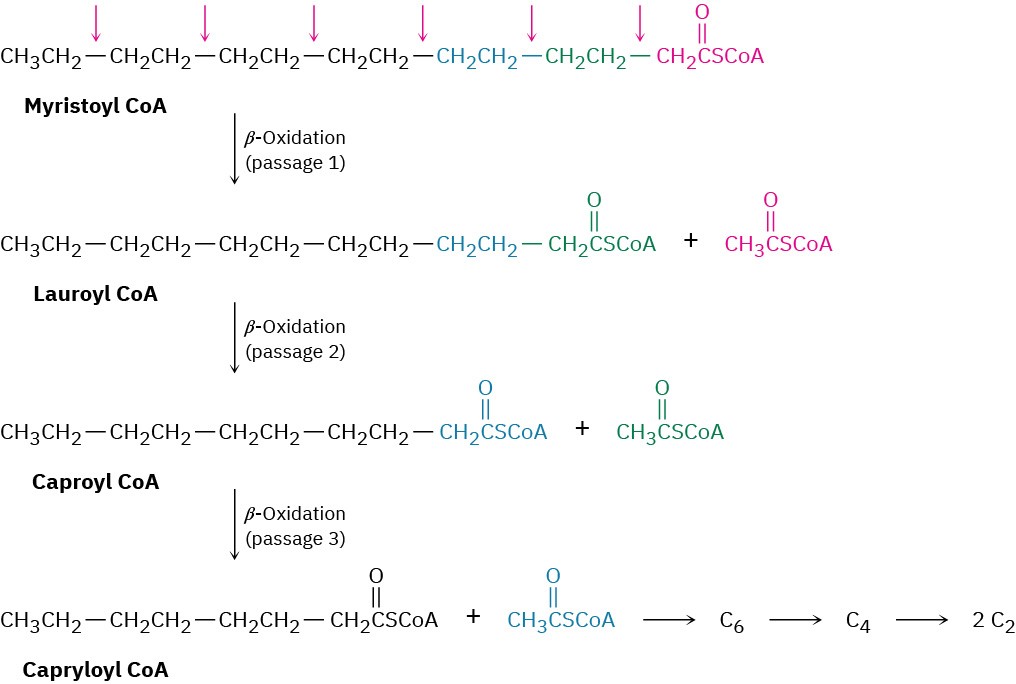
Figure 29.5Catabolism of the 14-carbon myristic acid by the β-oxidation pathway yields seven molecules of acetyl CoA after six passages.
Most fatty acids have an even number of carbon atoms, so none are left over after β– oxidation. Those fatty acids with an odd number of carbon atoms yield the three-carbon propionyl CoA in the final β-oxidation. Propionyl CoA is then converted to succinate by a multistep radical pathway, and succinate enters the citric acid cycle (Section 29.7). Note that the three-carbon propionyl group should technically be called propanoyl, but biochemists generally use the nonsystematic name.
Problem 29-2
Write the equations for the remaining passages of the β-oxidation pathway following those shown in Figure 29.5.
Problem 29-3
How many molecules of acetyl CoA are produced by catabolism of the following fatty acids, and how many passages of the β-oxidation pathway are needed?
(a)
Palmitic acid, CH3(CH2)14CO2H
(b)
Arachidic acid, CH3(CH2)18CO2H

