As practiced by organic chemists, NMR spectroscopy is a powerful method of structure determination. A small amount of sample, typically a few milligrams or less, is dissolved in a small amount of solvent, the solution is placed in a thin glass tube, and the tube is placed into the narrow (1–2 cm) gap between the poles of a strong magnet. Imagine, though, that a much larger NMR instrument were available. Instead of a few milligrams, the sample size could be tens of kilograms; instead of a narrow gap between magnet poles, the gap could be large enough for a whole person to climb into so that an NMR spectrum of body parts could be obtained. That large instrument is exactly what’s used for magnetic resonance imaging (MRI), a diagnostic technique of enormous value to the medical community.
Like NMR spectroscopy, MRI takes advantage of the magnetic properties of certain nuclei, typically hydrogen, and of the signals emitted when those nuclei are stimulated by radiofrequency energy. Unlike what happens in NMR spectroscopy, though, MRI instruments use data manipulation techniques to look at the three-dimensional location of magnetic nuclei in the body rather than at the chemical nature of the nuclei. As noted, most MRI instruments currently look at hydrogen, present in abundance wherever there is water or fat in the body.
The signals detected by MRI vary with the density of hydrogen atoms and with the nature of their surroundings, allowing identification of different types of tissue and even allowing the visualization of motion. For example, the volume of blood leaving the heart in a single stroke can be measured, and heart motion can be observed. Soft tissues that don’t show up well on X-ray images can be seen clearly, allowing diagnosis of brain tumors, strokes, and other conditions. This technique is also valuable in diagnosing damage to knees or other joints and is a noninvasive alternative to surgical explorations.
Several types of atoms in addition to hydrogen can be detected by MRI, and the applications of images based on 31P atoms are being explored. This approach holds great promise for studies of metabolism.
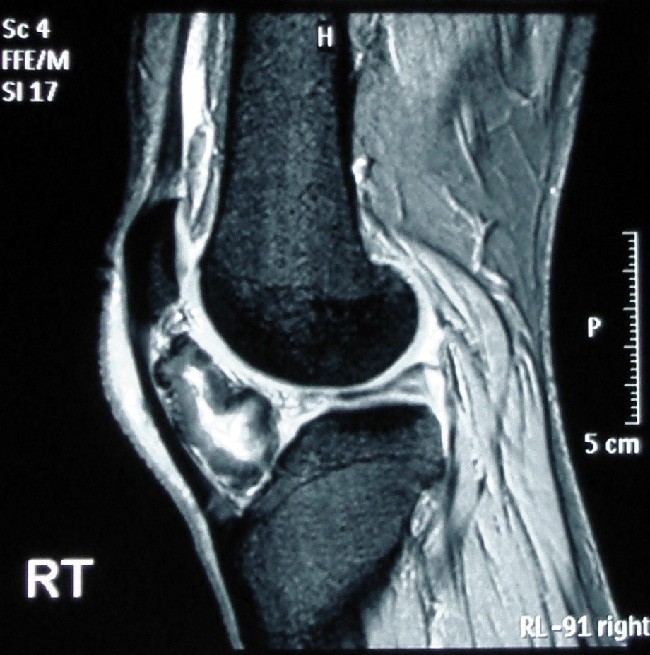
Figure 13.23 If you’re a runner, you really don’t want this to happen to you. The MRI of this left knee shows bone formation in the tissue in a patient with osteochondroma. (credit: “Osteochondroma MRI” by Michael R Carmont, Sian Davies, Daniel Gey van Pittius and Robin Rees/Wikimedia Commons, CC BY 2.0)

