Coronary heart disease—the buildup of cholesterol-containing plaques on the walls of heart arteries—is the leading cause of death for people older than 20 in industrialized countries. It’s estimated that up to one-third of women and one-half of men will develop the disease at some point in their lives.
The onset of coronary heart disease is directly correlated with blood cholesterol levels (see the Chapter 27 Chemistry Matters), and the first step in disease prevention is to lower those levels. It turns out that only about 25% of your blood cholesterol comes from what you eat; the remaining 75%—about 1 gram each day—is biosynthesized in your liver from dietary fats and carbohydrates. Thus, any effective plan for lowering your cholesterol level means limiting the amount that your body makes, which is where a detailed chemical knowledge of cholesterol biosynthesis comes in.
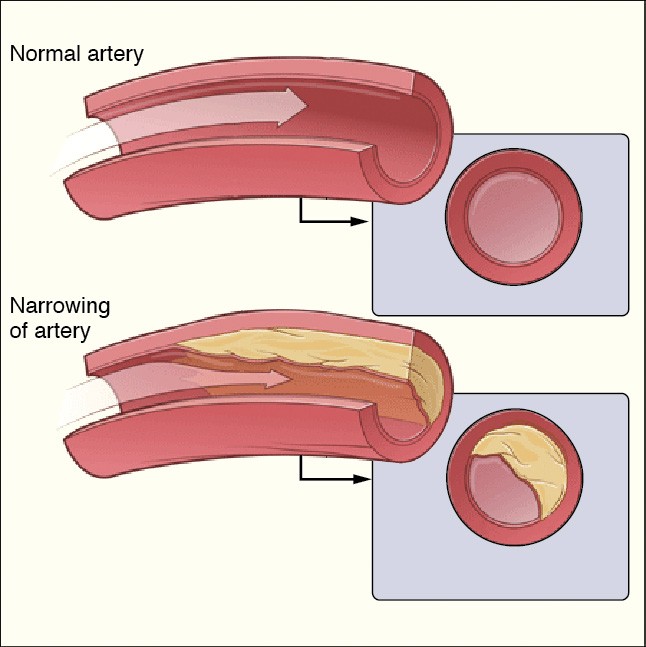
Figure 29.18The buildup of cholesterol deposits inside arteries can cause coronary heart disease, a leading cause of death for adults in industrialized nations.
We saw in Section 27.5 and Section 27.7 that all steroids, including cholesterol, are biosynthesized from the triterpenoid lanosterol, which in turn comes from acetyl CoA through isopentenyl diphosphate. If you knew all the mechanisms for all the chemical steps in cholesterol biosynthesis, you might be able to devise a drug that would block one of those steps, thereby short-circuiting the biosynthetic process and controlling the amount of cholesterol produced.
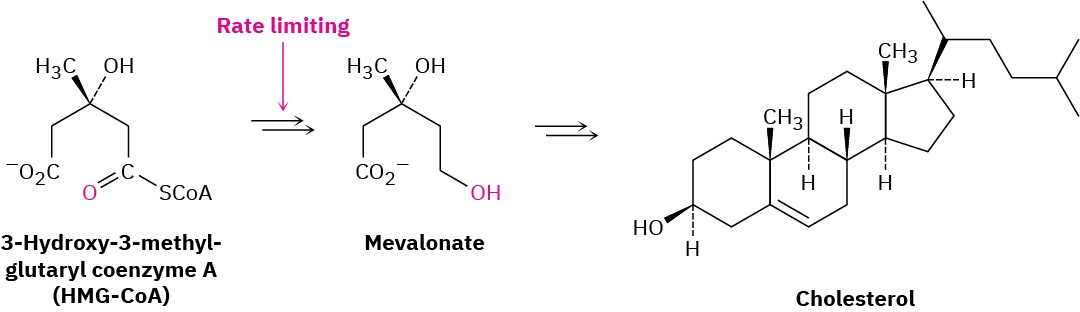
But we do know those mechanisms! Look back at the pathway for the biosynthesis of isopentenyl diphosphate from acetyl CoA, shown in Figure 27.8. It turns out that the rate- limiting step in the pathway is the reduction of 3-hydroxy-3-methylglutaryl CoA (abbreviated HMG-CoA) to mevalonate, brought about by the enzyme HMG-CoA reductase. If that enzyme could be stopped from functioning, cholesterol biosynthesis would also be stopped.
To find a drug that blocks HMG-CoA reductase, chemists did two simultaneous experiments on a large number of potential drug candidates isolated from soil microbes. In one experiment, the drug candidate and mevalonate were added to liver extract; in the second experiment, only the drug candidate was added without mevalonate. If cholesterol was produced only in the presence of added mevalonate but not in the absence of mevalonate, the drug candidate must have blocked the enzyme for mevalonate synthesis.
The drugs that block HMG-CoA reductase, and thus control cholesterol synthesis in the body, are called statins. In just the 10-year period following their introduction in 1994, the death rate from coronary heart disease decreased by 33% in the United States.
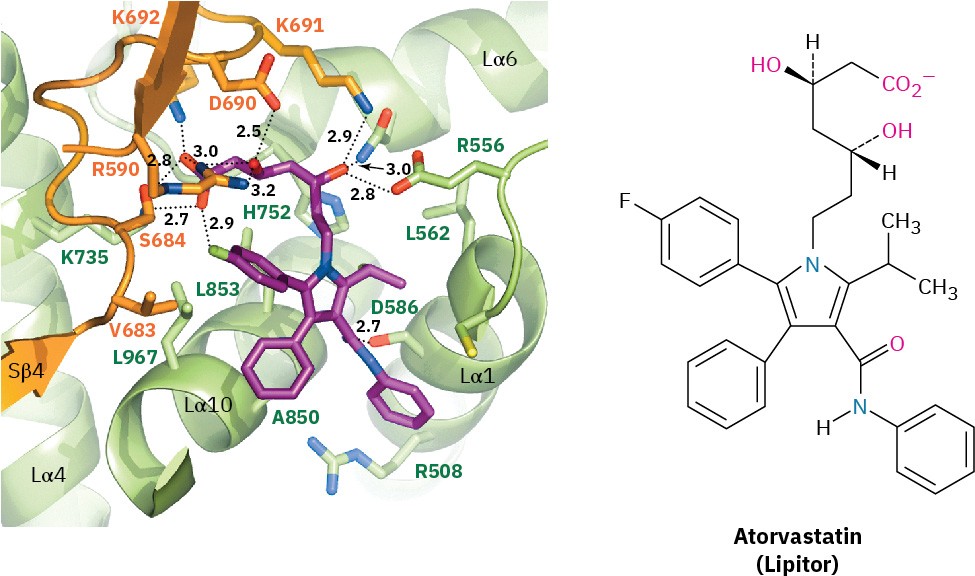
Like many drugs, statins don’t come without risks and have some serious side effects that people considering their use should be aware of, but approaching 30 years later, they remain among the most widely prescribed drugs in the world, with estimated annual sales of $14 billion. Atorvastatin (Lipitor), simvastatin (Zocor), rosuvastatin (Crestor), pravastatin (Pravachol), and lovastatin (Mevacor) are some examples. An X-ray crystal structure of the active site in the HMG-CoA reductase enzyme is shown in the accompanying graphic, along with a molecule of atorvastatin (purple) that is tightly bound in the active site and stops the enzyme from functioning. A good understanding of organic chemistry certainly paid off in this instance.