It has been estimated that major pharmaceutical companies in the United States spent some $200 billion on drug research and development in 2020, while government agencies and private foundations spent another $28 billion. What does this money buy? From 1983 to 2022, the money resulted in a total of 1237 new molecular entities (NMEs)—new biologically active chemical substances approved for sale as drugs by the U.S. Food and Drug Administration (FDA).
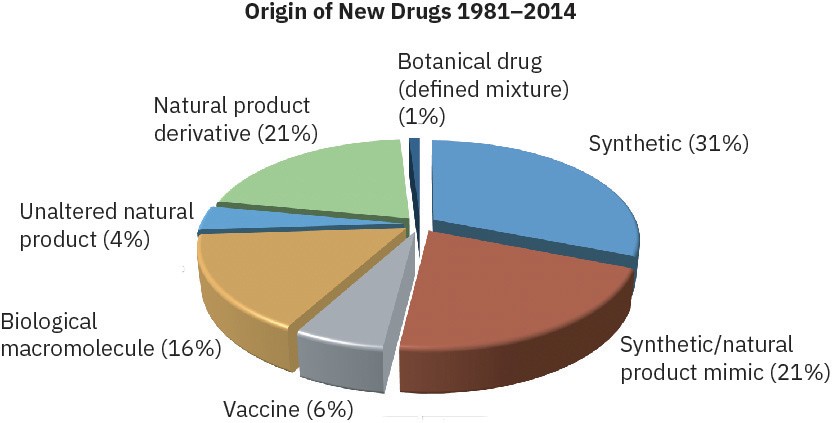
Where do the new drugs come from? According to a study carried out several years ago at the U.S. National Cancer Institute, only about 33% of new drugs are entirely synthetic and completely unrelated to any naturally occurring substance. The remaining 67% take their lead, to a greater or lesser extent, from nature. Vaccines and genetically engineered proteins of biological origin account for 15% of NMEs, but most new drugs come from natural products, a catchall term generally taken to mean small molecules found in bacteria, plants, algae, and other living organisms. Unmodified natural products isolated directly from the producing organism account for 24% of NMEs, while natural products that have been chemically modified in the laboratory account for the remaining 28%.
Many years of work go into screening many thousands of substances to identify a single compound that might ultimately gain approval as an NME. But after that single compound has been identified, the work has just begun because it takes an average of 9 to 10 years for a drug to make it through the approval process. First, the safety of the drug in animals must be demonstrated and an economical method of manufacture must be devised. With these preliminaries out of the way, an Investigational New Drug (IND) application is submitted to the FDA for permission to begin testing in humans.

Figure 6.11 Introduced in June, 2006, Gardasil is the first vaccine ever approved for the prevention of cancer. Where do new drugs like this come from? (credit: modification of work “COVIran Barekat vaccine production” by Sadegh Nikgostar/Wikimedia Commons, CC BY 4.0)
Human testing takes, or should take, 5 to 7 years and is divided into three phases. Phase I clinical trials are carried out on a small group of healthy volunteers to establish safety and look for side effects. Several months to a year are needed, and only about 70% of drugs pass at this point. Phase II clinical trials next test the drug for 1 to 2 years in several hundred patients with the target disease or condition, looking both for safety and efficacy, and only about 33% of the original group pass. Finally, phase III trials are undertaken on a large sample of patients to document definitively the drug’s safety, dosage, and efficacy. If the drug is one of the 25% of the original group that make it to the end of phase III, all the data are then gathered into a New Drug Application (NDA) and sent to the FDA for review and approval, which can take another 2 years. Ten years have elapsed and at least $500 million has been spent, with only a 20% success rate for the drugs that began testing.
Finally, though, the drug will begin to appear in medicine cabinets. The following timeline shows the process.