Amides, like esters, are abundant in all living organisms. Proteins, nucleic acids, and many pharmaceutical agents have amide functional groups. The reason for this abundance of amides is that they are stable in the aqueous conditions found in living organisms. Amides are the least reactive of the common acid derivatives and undergo relatively few nucleophilic acyl substitution reactions.
Preparation of Amides
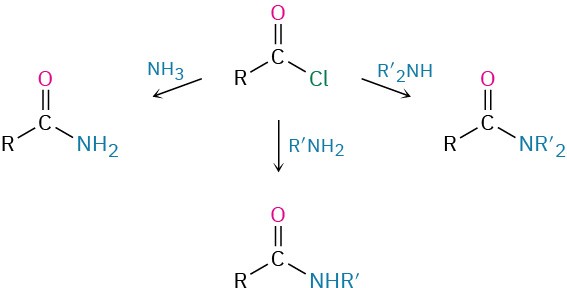
Amides are usually prepared by reaction of an amine with an acid chloride (Section 21.4). Ammonia, monosubstituted amines, and disubstituted amines all undergo the reaction.
Reactions of Amides
Conversion of Amides into Carboxylic Acids: Hydrolysis
Amides undergo hydrolysis to yield carboxylic acids plus ammonia or an amine upon heating in either aqueous acid or aqueous base. The conditions required for amide hydrolysis are more extreme than those required for the hydrolysis of acid chlorides or esters, but the mechanisms are similar. Acidic hydrolysis reaction occurs by nucleophilic addition of water to the protonated amide, followed by transfer of a proton from oxygen to nitrogen to make the nitrogen a better leaving group, and subsequent elimination. The steps are reversible, with the equilibrium shifted toward product by protonation of NH3 in the final step.
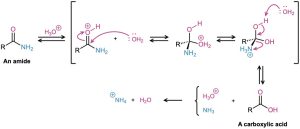
Basic hydrolysis occurs by nucleophilic addition of OH– to the amide carbonyl group, followed by elimination of amide ion (–NH2) and subsequent deprotonation of the initially formed carboxylic acid by ammonia. The steps are reversible, with the equilibrium shifted toward product by the final deprotonation of the carboxylic acid. Basic hydrolysis is substantially more difficult than the analogous acid-catalyzed reaction because amide ion is a very poor leaving group, making the elimination step difficult.
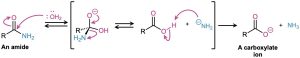
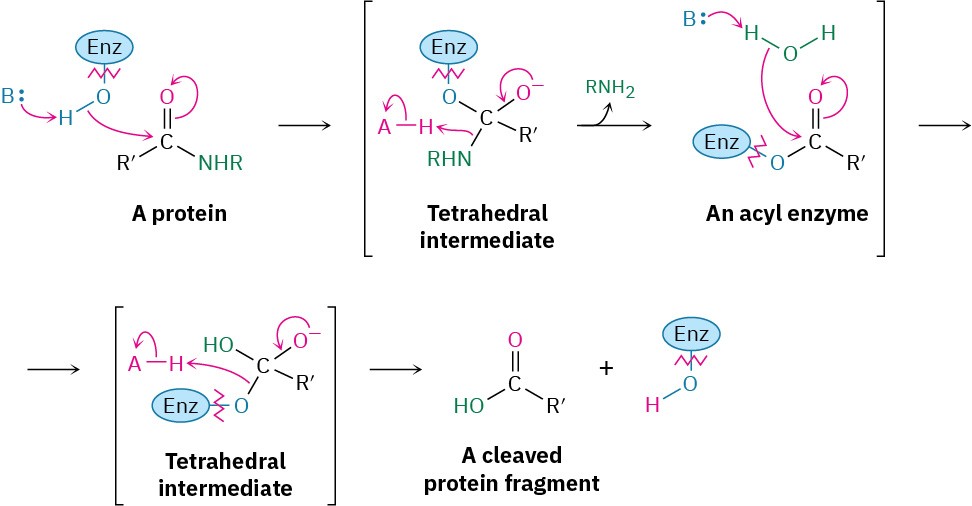
Amide hydrolysis is common in biological chemistry. Just as the hydrolysis of esters is the initial step in the digestion of dietary fats, the hydrolysis of amides is the initial step in the digestion of dietary proteins. The reaction is catalyzed by protease enzymes and occurs by a mechanism almost identical to what we just saw for fat hydrolysis. That is, an initial nucleophilic acyl substitution of an alcohol group in the enzyme on an amide linkage in the protein gives an acyl enzyme intermediate, which then undergoes hydrolysis.
Conversion of Amides into Amines: Reduction
Like other carboxylic acid derivatives, amides can be reduced by LiAlH4. The product of the reduction, however, is an amine rather than an alcohol. The net effect of an amide reduction is thus the conversion of the amide carbonyl group into a methylene group (C=O → CH2). This kind of reaction is specific to amides and does not occur with other carboxylic acid derivatives.
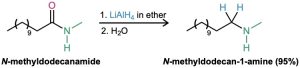
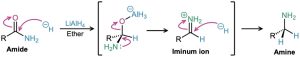
Amide reduction occurs by nucleophilic addition of hydride ion to the amide carbonyl group, followed by expulsion of the oxygen atom as an aluminate anion leaving group to give an iminium ion intermediate. The intermediate iminium ion is further reduced by LiAlH4 to yield the amine.
The reaction is effective with both acyclic and cyclic amides, or lactams, and is a good method for preparing cyclic amines.
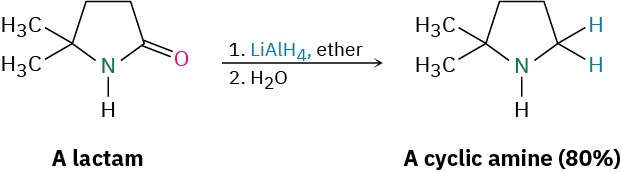
Worked Example 21.4 Synthesizing an Amine from an Amide
How could you prepare N-ethylaniline by reduction of an amide with LiAlH4?
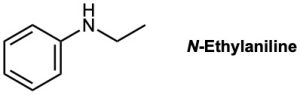
Strategy
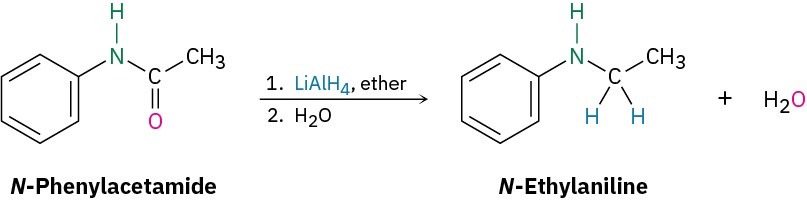
Reduction of an amide with LiAlH4 yields an amine. To find the starting material for synthesis of N-ethylaniline, look for a CH2 position next to the nitrogen atom and replace that CH2 by C═O. In this case, the amide is N-phenylacetamide.
Solution
Problem 21-20
How would you convert N-ethylbenzamide to each of the following products?
(a) benzoic acid
(b) benzyl alcohol
(c) benzyl ethylamine
Problem 21-21
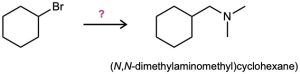
How would you use the reaction of an amide with LiAlH4 as the key step in going from bromocyclohexane to (N,N-dimethylaminomethyl)cyclohexane? Write all the steps in the reaction sequence.