As mentioned in the chapter introduction, the substrate for a nucleophilic acyl substitution reaction in living organisms is generally either a thioester (RCOSR′) or an acyl phosphate (RCO2PO32– or RCO2PO3R′–). Neither is as reactive as an acid chloride or acid anhydride, yet both are stable enough to exist in living organisms while still reactive enough to undergo acyl substitution.
Acyl CoA’s, such as acetyl CoA, are the most common thioesters in nature. Coenzyme A, abbreviated CoA, is a thiol formed by a phosphoric anhydride linkage (O═P–O–P═O) between phosphopantetheine and adenosine 3′,5′-bisphosphate. (The prefix bis– means “two” and indicates that adenosine 3′,5′-bisphosphate has two phosphate groups, one on C3′ and one on C5′.) Reaction of coenzyme A with an acyl phosphate or acyl adenylate gives acyl CoA (Figure 21.10). As we saw in Section 21.3 (Figure 21.7), formation of the acyl adenylate occurs by reaction of a carboxylic acid with ATP and is itself a nucleophilic acyl substitution reaction that takes place on phosphorus.
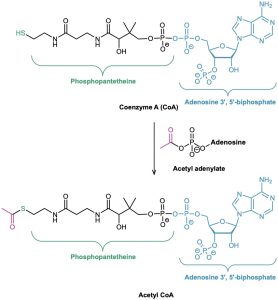
Figure 21.10 Formation of the thioester acetyl CoA by nucleophilic acyl substitution reaction of coenzyme A (CoA) with acetyl adenylate.
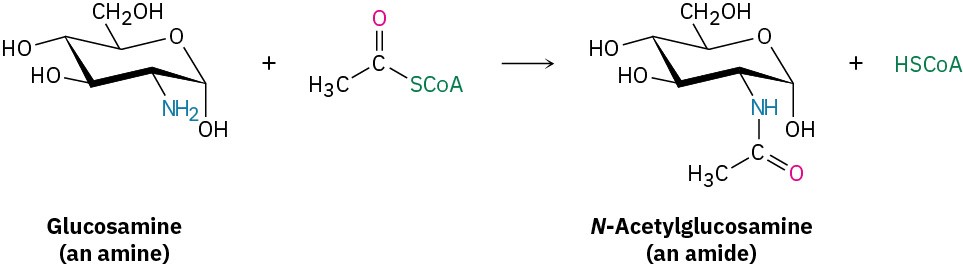
Once formed, an acyl CoA is a substrate for further nucleophilic acyl substitution reactions. For example, N-acetylglucosamine, a component of cartilage and other connective tissues, is synthesized by an aminolysis reaction between glucosamine and acetyl CoA.
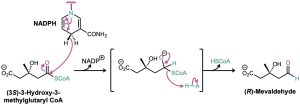
Another example of a nucleophilic acyl substitution reaction on a thioester—this one a substitution by hydride ion to effect the partial reduction of a thioester to an aldehyde— occurs in the biosynthesis of mevaldehyde, an intermediate in terpenoid synthesis, which we’ll discuss in some detail in Section 27.5. In this reaction, (3S)-3-hydroxy-3-methylglutaryl CoA is reduced by hydride donation from NADPH.
Problem 21-22
Write the mechanism of the reaction shown in Figure 21.10 between coenzyme A and acetyl adenylate to give acetyl CoA.

