We saw in the chapter on Structure and Bonding that the carbon–carbon double bond can be described in two ways. In valence bond language (Section 1.8), the carbons are sp2– hybridized and have three equivalent hybrid orbitals that lie in a plane at angles of 120° to one another. The carbons form a σ bond by a head-on overlap of sp2 orbitals and form a π bond by sideways overlap of unhybridized p orbitals oriented perpendicular to the sp2 plane, as shown in Figure 1.15.
In molecular orbital language (Section 1.11), interaction between the p orbitals leads to one bonding and one antibonding π molecular orbital. The π bonding MO has no node between nuclei and results from a combination of p orbital lobes with the same algebraic sign. The π antibonding MO has a node between nuclei and results from a combination of lobes with different algebraic signs, as shown in Figure 1.19.
Although essentially free rotation around single bonds is possible (Section 3.6), the same is not true of double bonds. For rotation to occur around a double bond, the π bond must break and re-form (Figure 7.3). Thus, the barrier to double-bond rotation must be at least as great as the strength of the π bond itself, an estimated 350 kJ/mol. Recall that the barrier to bond rotation in ethane is only 12 kJ/mol.
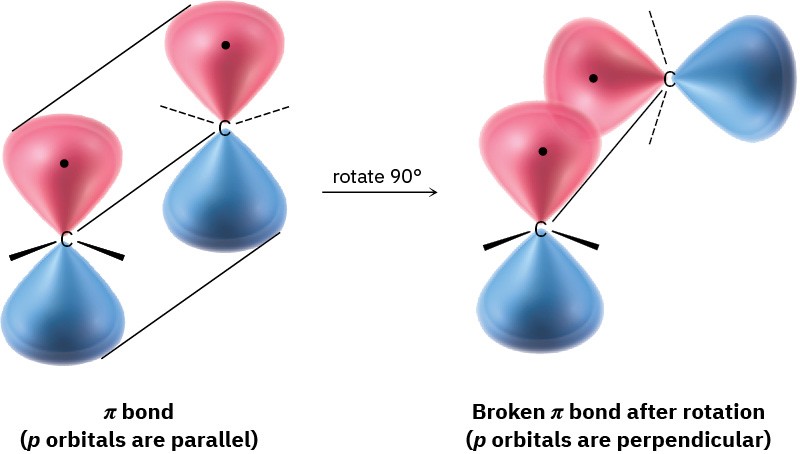
Figure 7.3 The π bond must break for rotation to take place around a carbon– carbon double bond.
The lack of rotation around carbon–carbon double bonds is of more than just theoretical interest; it also has chemical consequences. Imagine the situation for a disubstituted alkene such as 2-butene. (Disubstituted means that two substituents other than hydrogen are bonded to the double-bond carbons.) The two methyl groups in 2-butene can either be on the same side of the double bond or on opposite sides, a situation similar to that in disubstituted cycloalkanes (Section 4.2).
Since bond rotation can’t occur, the two 2-butenes can’t spontaneously interconvert; they are different, isolable compounds. As with disubstituted cycloalkanes, we call such
compounds cis–trans stereoisomers. The compound with substituents on the same side of the double bond is called cis-2-butene, and the isomer with substituents on opposite sides is trans-2-butene (Figure 7.4).
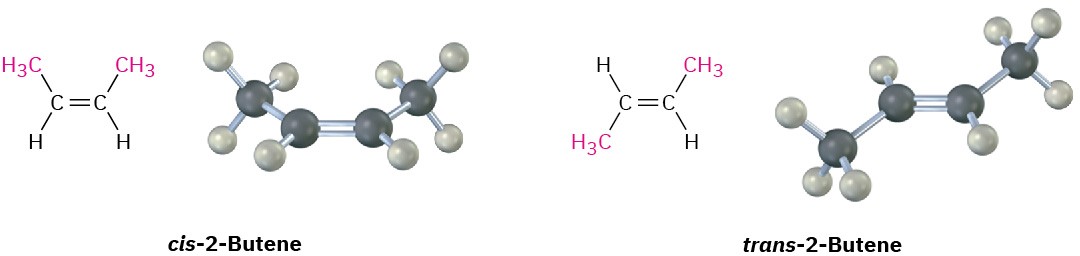
Figure 7.4 Cis and trans isomers of 2-butene. The cis isomer has the two methyl groups on the same side of the double bond, and the trans isomer has methyl groups on opposite sides.
Cis–trans isomerism is not limited to disubstituted alkenes. It can occur whenever both double-bond carbons are attached to two different groups. If one of the double-bond carbons is attached to two identical groups, however, cis–trans isomerism is not possible (Figure 7.5).
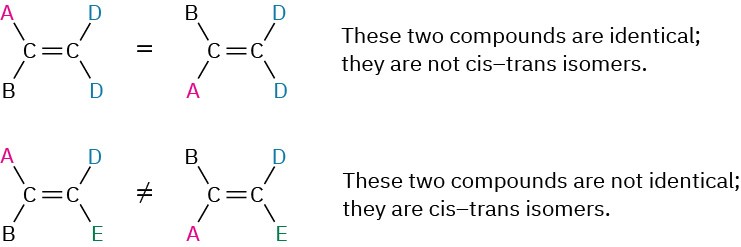
Figure 7.5 The requirement for cis–trans isomerism in alkenes. Compounds that have one of their carbons bonded to two identical groups can’t exist as cis–trans isomers. Only when both carbons are bonded to two different groups is cis–trans isomerism possible.
Problem 7-8
The sex attractant of the common housefly is an alkene named cis-9-tricosene. Draw its structure. (Tricosane is the straight-chain alkane C23H48.)
Problem 7-9
Which of the following compounds can exist as pairs of cis–trans isomers? Draw each pair, and indicate the geometry of each isomer.
(a) CH3CH=CH2
(b) (CH3)2C=CHCH3
(c) CH3CH2CH=CHCH3
(d) (CH3)2C=C(CH3)CH2CH3
(e) ClCH=CHCl
(f) BrCH=CHCl
Problem 7-10
Name the following alkenes, including a cis or trans designation:
(a)
(b)