In many respects, the chemistry of cycloalkanes is like that of open-chain alkanes: both are nonpolar and fairly inert. There are, however, some important differences. One difference is that cycloalkanes are less flexible than open-chain alkanes. In contrast with the relatively free rotation around single bonds in open-chain alkanes, there is much less freedom in cycloalkanes. Cyclopropane, for example, must be a rigid planar molecule because three points (the carbon atoms) define a plane. No bond rotation can take place around a cyclopropane carbon–carbon bond without breaking open the ring (Figure 4.2).
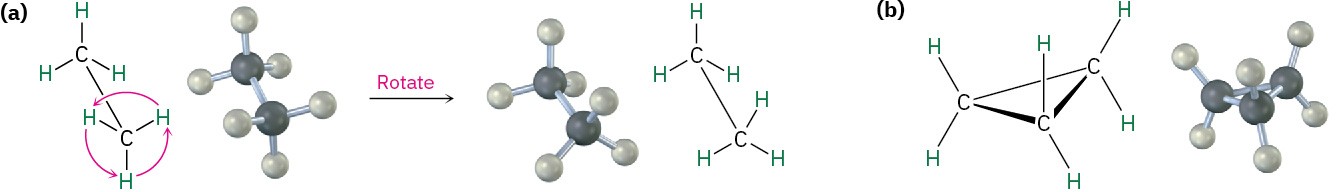
Figure 4.2 Bond rotation in ethane and cyclopropane. (a) Rotation occurs around the carbon–carbon bond in ethane, but (b) no rotation is possible around the carbon–carbon bonds in cyclopropane without breaking open the ring.
Larger cycloalkanes have increasing rotational freedom, and very large rings (C25 and up) are so floppy that they are nearly indistinguishable from open-chain alkanes. The common ring sizes (C3–C7), however, are severely restricted in their molecular motions.
Because of their cyclic structures, cycloalkanes have two faces when viewed edge-on, a “top” face and a “bottom” face. As a result, isomerism is possible in substituted cycloalkanes. For example, there are two different 1,2-dimethylcyclopropane isomers, one with the two methyl groups on the same face of the ring and one with the methyl groups on opposite faces (Figure 4.3). Both isomers are stable compounds, and neither can be converted into the other without breaking and reforming chemical bonds.
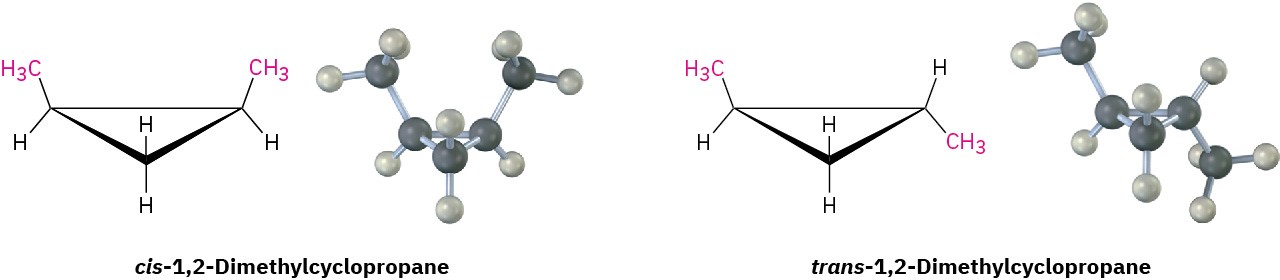
Figure 4.3 There are two different 1,2-dimethylcyclopropane isomers, one with the methyl groups on the same face of the ring (cis) and the other with the methyl groups on opposite faces of the ring (trans). The two isomers do not interconvert.
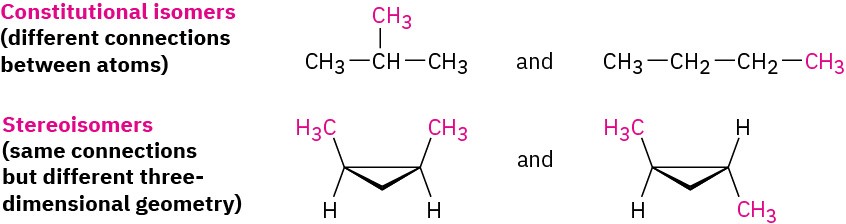
Unlike the constitutional isomers butane and isobutane, which have their atoms connected in a different order, the two 1,2-dimethylcyclopropanes have the same order of connections but differ in the spatial orientation of the atoms. Such compounds, with atoms connected in the same order but differing in three-dimensional orientation, are called stereochemical isomers, or stereoisomers. As we saw in Section 3.6, the term stereochemistry is used generally to refer to the three-dimensional aspects of structure and reactivity.
The 1,2-dimethylcyclopropanes are members of a subclass of stereoisomers called cis– trans isomers. The prefixes cis– (Latin “on the same side”) and trans– (Latin “across”) are used to distinguish between them. Cis–trans isomerism is a common occurrence in substituted cycloalkanes and in many cyclic biological molecules.
Worked Example 4.1
Naming Cycloalkanes
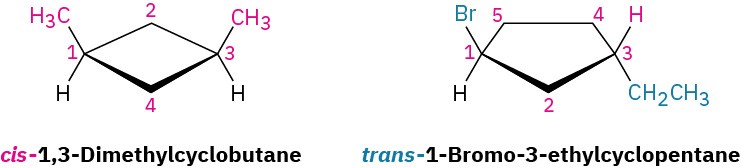
Name the following substances, including the cis- or trans- prefix:
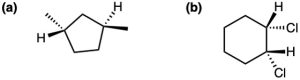
Strategy
In these views, the ring is roughly in the plane of the page, a wedged bond protrudes out of the page, and a dashed bond recedes into the page. Two substituents are cis if they are both out of or both into the page, and they are trans if one is out of and one is into the page.
Solution trans-1,3-Dimethylcyclopentane cis-1,2-Dichlorocyclohexane
Problem 4-4
Name the following substances, including the cis– or trans– prefix:
(a) 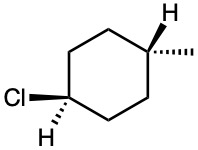
(b) 
Problem 4-5
Draw the structures of the following molecules:
(a) trans-1-Bromo-3-methylcyclohexane
(b) cis-1,2-Dimethylcyclobutane
(c) trans-1-tert-Butyl-2-ethylcyclohexane
Problem 4-6
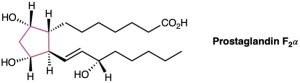
Prostaglandin F2α, a hormone that causes uterine contraction during childbirth, has the following structure. Are the two hydroxyl groups (–OH) on the cyclopentane ring cis or trans to each other? What about the two carbon chains attached to the ring?
Problem 4-7
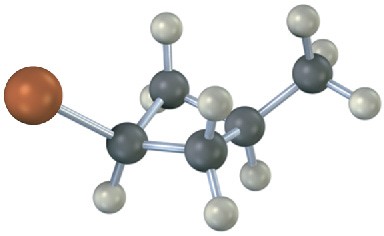
Name the following substances, including the cis– or trans– prefix (red-brown = Br):
(a) 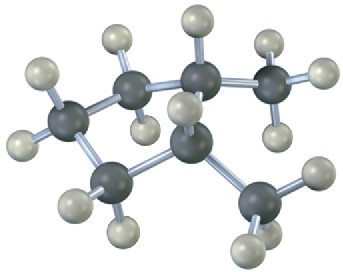
(b)