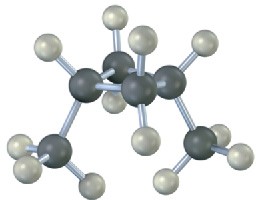
Cyclopropane
Cyclopropane is the most strained of all rings, primarily due to the angle strain caused by its 60° C−C−C bond angles. In addition, cyclopropane has considerable torsional strain because the C−H bonds on neighboring carbon atoms are eclipsed (Figure 4.5).
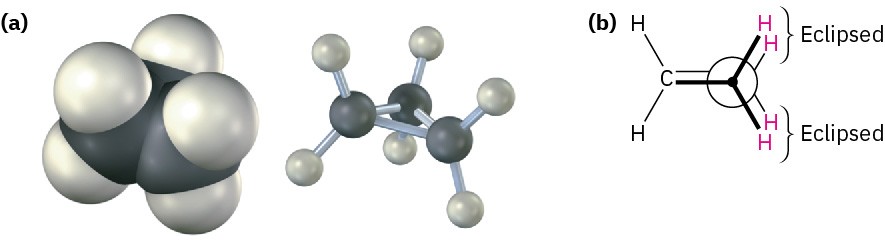
Figure 4.5 The structure of cyclopropane, showing the eclipsing of neighboring C−H bonds that gives rise to torsional strain. Part (b) is a Newman projection along a C−C bond.
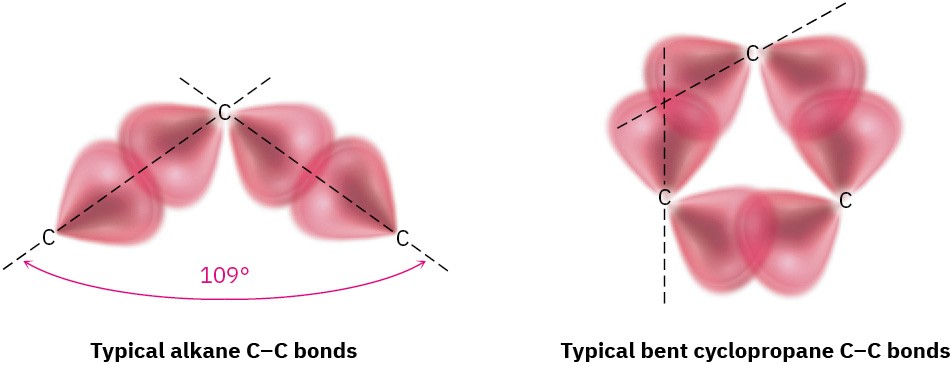
How can the hybrid-orbital model of bonding account for the large distortion of bond angles from the normal 109° tetrahedral value to 60° in cyclopropane? The answer is that cyclopropane has bent bonds. In an unstrained alkane, maximum bonding is achieved when two atoms have their overlapping orbitals pointing directly toward each other. In cyclopropane, though, the orbitals can’t point directly toward each other; instead, they overlap at a slight angle. The result is that cyclopropane bonds are weaker and more reactive than typical alkane bonds: 255 kJ/mol (61 kcal/mol) for a C−C bond in cyclopropane versus 370 kJ/mol (88 kcal/mol) for a C−C bond in open-chain propane.
Cyclobutane
Cyclobutane has less angle strain than cyclopropane but has more torsional strain because of its larger number of ring hydrogens. As a result, the total strain for the two compounds is nearly the same: 110 kJ/mol (26.4 kcal/mol) for cyclobutane versus 115 kJ/mol (27.5 kcal/mol) for cyclopropane. Cyclobutane is not quite flat but is slightly bent so that one carbon atom lies about 25° above the plane of the other three (Figure 4.6). The effect of this slight bend is to increase angle strain but to decrease torsional strain, until a minimum- energy balance between the two opposing effects is achieved.
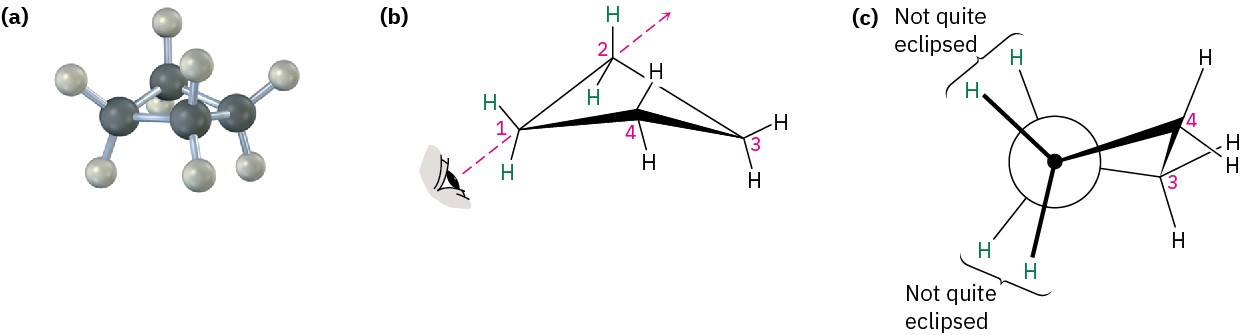
Figure 4.6 The conformation of cyclobutane. Part (c) is a Newman projection along a C−C bond, showing that neighboring C−H bonds are not quite eclipsed.
Cyclopentane
Cyclopentane was predicted to be nearly strain-free, but it actually has a total strain energy of 26 kJ/mol (6.2 kcal/mol). Although planar cyclopentane has practically no angle strain, it has a large torsional strain. Cyclopentane therefore twists to adopt a puckered, nonplanar conformation that strikes a balance between increased angle strain and decreased torsional strain. Four of the cyclopentane carbon atoms are in approximately the same plane, with the fifth carbon atom bent out of the plane. Most of the hydrogens are nearly staggered with respect to their neighbors (Figure 4.7).
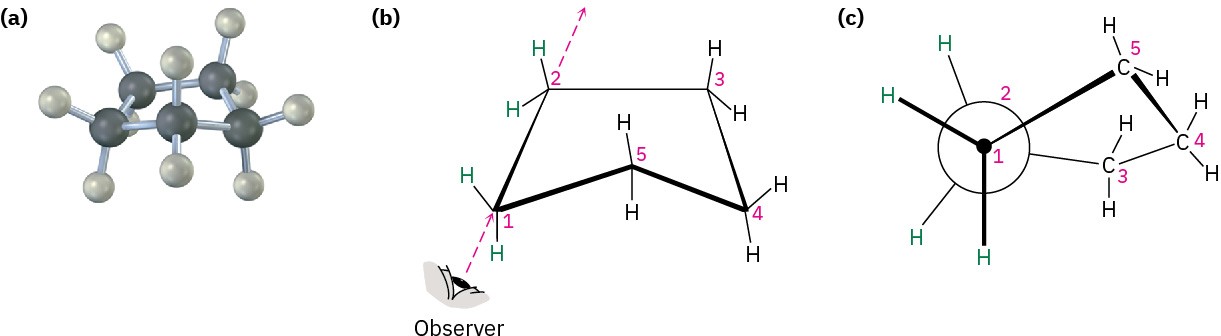
Figure 4.7 The conformation of cyclopentane. Carbons 1, 2, 3, and 4 are nearly coplanar, but carbon 5 is out of the plane. Part (c) is a Newman projection along the C1–C2 bond, showing that neighboring C−H bonds are nearly staggered.
Problem 4-10
How many H ⟷ H eclipsing interactions would be present if cyclopentane were planar? Assuming an energy cost of 4.0 kJ/mol for each eclipsing interaction, how much torsional strain would planar cyclopentane have? Since the measured total strain of cyclopentane is 26 kJ/mol, how much of the torsional strain is relieved by puckering?
Problem 4-11
Two conformations of cis-1,3-dimethylcyclobutane are shown. What is the difference between them, and which do you think is likely to be more stable?
(a)  (b)
(b)