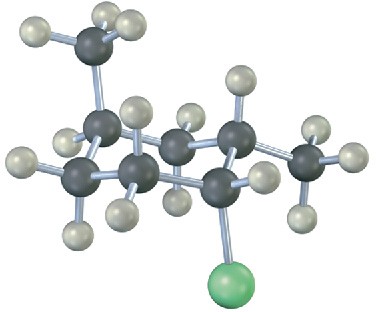
Monosubstituted cyclohexanes are always more stable with their substituent in an equatorial position, but the situation with disubstituted cyclohexanes is more complex because the steric effects of both substituents must be taken into account. All steric interactions for both possible chair conformations must be analyzed before deciding which conformation is favored.
Let’s look at 1,2-dimethylcyclohexane as an example. There are two isomers, cis-1,2- dimethylcyclohexane and trans-1,2-dimethylcyclohexane, which must be considered separately. In the cis isomer, both methyl groups are on the same face of the ring and the compound can exist in either of the two chair conformations shown in Figure 4.16.
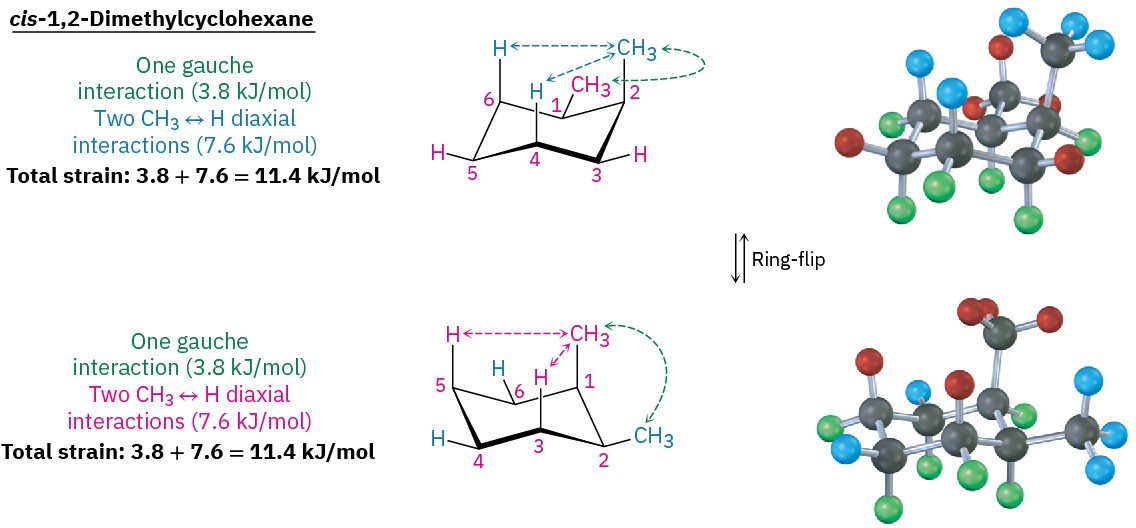
Figure 4.16 Conformations of cis-1,2-dimethylcyclohexane. The two chair conformations are equal in energy because each has one axial methyl group and one equatorial methyl group.
Both chair conformations of cis-1,2-dimethylcyclohexane have one axial methyl group and one equatorial methyl group. The top conformation in Figure 4.16 has an axial methyl group at C2, which has 1,3-diaxial interactions with hydrogens on C4 and C6. The ring-flipped conformation has an axial methyl group at C1, which has 1,3-diaxial interactions with hydrogens on C3 and C5. In addition, both conformations have gauche butane interactions between the two methyl groups. The two conformations are equal in energy, with a total steric strain of 3 × 3.8 kJ/mol = 11.4 kJ/mol (2.7 kcal/mol).
In trans-1,2-dimethylcyclohexane, the two methyl groups are on opposite sides of the ring and the compound can exist in either of the two chair conformations shown in Figure 4.17. The situation here is quite different from that of the cis isomer. The top conformation in Figure 4.17 has both methyl groups equatorial with only a gauche butane interaction between them (3.8 kJ/mol) but no 1,3-diaxial interactions. The ring-flipped conformation, however, has both methyl groups axial. The axial methyl group at C1 interacts with axial hydrogens at C3 and C5, and the axial methyl group at C2 interacts with axial hydrogens at C4 and C6. These four 1,3-diaxial interactions produce a steric strain of 4 × 3.8 kJ/mol = 15.2 kJ/mol and make the diaxial conformation 15.2 − 3.8 = 11.4 kJ/mol less favorable than the diequatorial conformation. We therefore predict that trans-1,2- dimethylcyclohexane will exist almost exclusively in the diequatorial conformation.
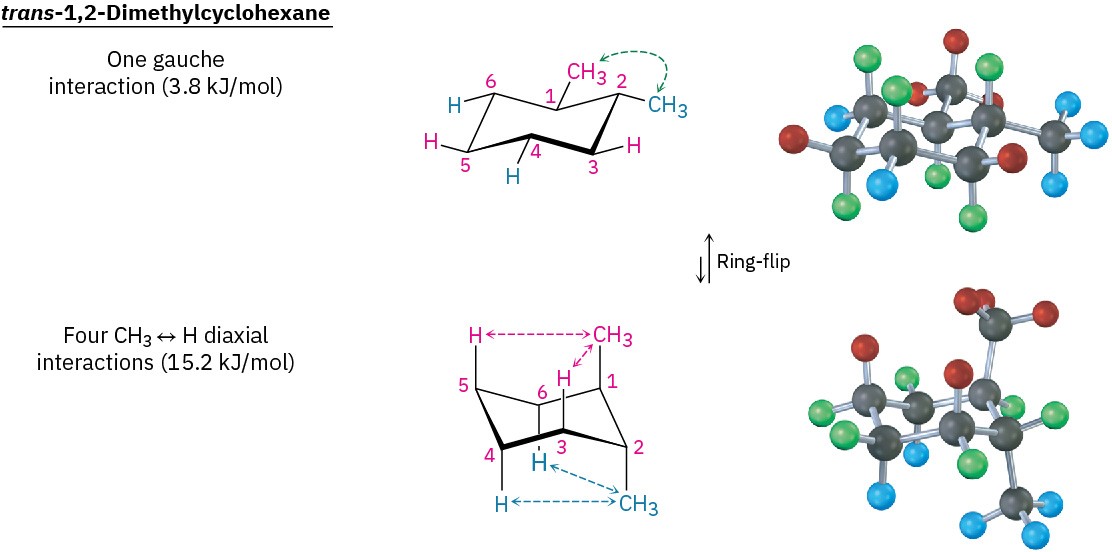
Figure 4.17 Conformations of trans-1,2-dimethylcyclohexane. The conformation with both methyl groups equatorial (top) is favored by 11.4 kJ/mol (2.7 kcal/mol) over the conformation with both methyl groups axial (bottom).
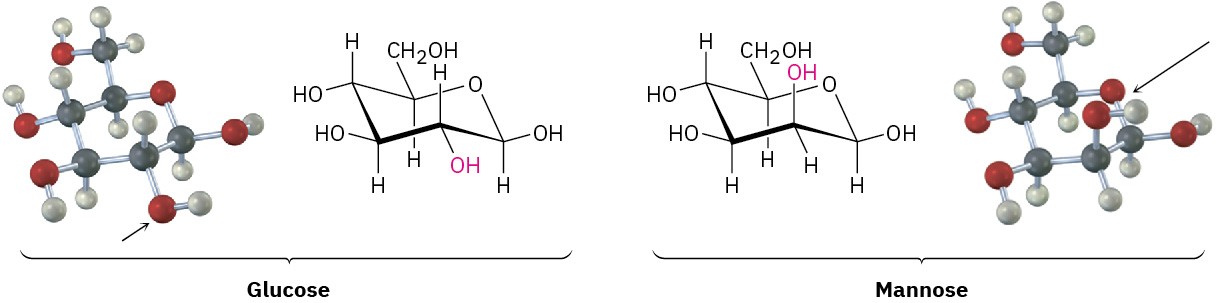
The same kind of conformational analysis just carried out for cis– and trans-1,2- dimethylcyclohexane can be done for any substituted cyclohexane, such as cis-1-tert-butyl- 4-chlorocyclohexane (see Worked Example 4.3). As you might imagine, though, the situation becomes more complex as the number of substituents increases. For instance, compare glucose with mannose, a carbohydrate present in seaweed. Which do you think is more strained? In glucose, all substituents on the six-membered ring are equatorial, while in mannose, one of the −OH groups is axial, making it more strained.
Worked Example 4.3
Drawing the Most Stable Conformation of a Substituted Cyclohexane
Draw the more stable chair conformation of cis-1-tert-butyl-4-chlorocyclohexane.
Strategy
Draw the two possible chair conformations and determine which is the more stable conformation. Remember that equatorial substituents cause less strain than axial substituents.
Solution
First draw the two chair conformations of the molecule:
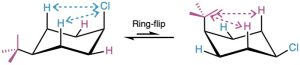
In the conformation on the left, the tert-butyl group is equatorial and the chlorine is axial. In the conformation on the right, the tert-butyl group is axial and the chlorine is equatorial. These conformations aren’t of equal energy because an axial tert-butyl substituent and an axial chloro substituent produce different amounts of steric strain. Table 4.1 shows that the 1,3-diaxial interaction between a hydrogen and a tert-butyl group costs 11.4 kJ/mol (2.7 kcal/mol), whereas the interaction between a hydrogen and a chlorine costs only 1.0 kJ/mol (0.25 kcal/mol). An axial tert-butyl group therefore produces (2 × 11.4 kJ/mol) − (2× 1.0 kJ/mol) = 20.8 kJ/mol (4.9 kcal/mol) more steric strain than an axial chlorine, and the compound preferentially adopts the conformation with the chlorine axial and the tert-butyl equatorial.
Problem 4-18
Draw the more stable chair conformation of the following molecules:
(a) trans-1-chloro-3-methylcyclohexane
(b) cis-1-ethyl-2-methylcyclohexane
(c) cis-1-bromo-4-ethylcyclohexane
(d) cis-1-tert-butyl-4-ethylcyclohexane
Problem 4-19
Identify each substituent in the following compound as axial or equatorial, and tell whether the conformation shown is the more stable or less stable chair form (green = Cl):