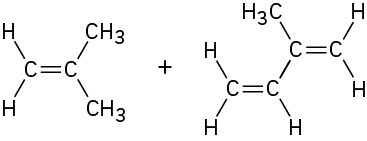
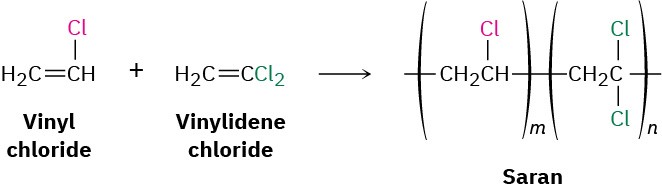
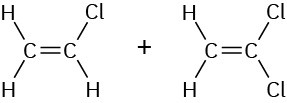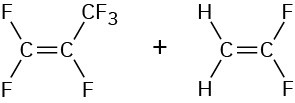Up to this point we’ve discussed only homopolymers—polymers that are made up of identical repeating units. In practice, however, copolymers are more important commercially. Copolymers are obtained when two or more different monomers are allowed to polymerize together. For example, copolymerization of vinyl chloride with vinylidene chloride (1,1-dichloroethylene) in a 1 : 4 ratio leads to the polymer Saran.
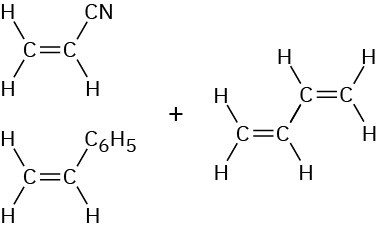
Copolymerization of monomer mixtures often leads to materials with properties quite different from those of either corresponding homopolymer, giving the polymer chemist a vast flexibility for devising new materials. Table 31.1 lists some common copolymers and their commercial applications.
Table 31.1 Some Common Copolymers and Their Uses
|
Monomers |
Structures |
Trade name |
Uses |
|
Vinyl chloride Vinylidene chloride |
|
Saran |
Fibers, food packaging |
|
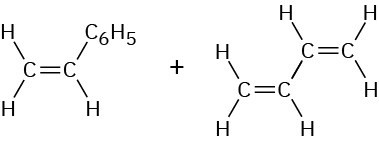
Styrene 1,3-Butadiene |
|
SBR (styrene– butadiene rubber) |
Tires, rubber articles |
|
Hexafluoropropene Vinylidene fluoride |
|
Viton |
Gaskets, seals |
|
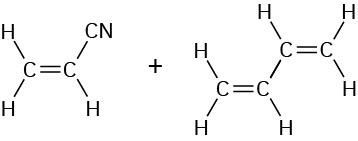
Acrylonitrile 1,3-Butadiene |
|
Nitrile rubber |
Adhesives, hoses |
|
Isobutylene Isoprene |
|
Butyl rubber |
Inner tubes |
|
Structures |
Trade name |
Uses |
|
|
Acrylonitrile 1,3-Butadiene Styrene |
|
ABS (monomer initials) |
Pipes, high-impact applications |
Several different types of copolymers can be defined, depending on the distribution of monomer units in the chain. If monomer A is copolymerized with monomer B, for instance, the resultant product might have a random distribution of the two units throughout the chain, or it might have an alternating distribution.
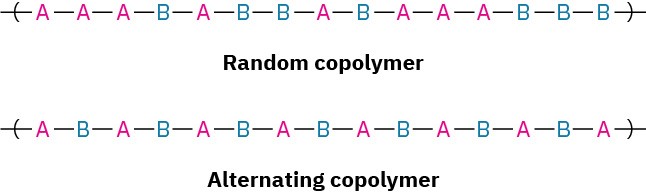
The exact distribution of monomer units depends on the initial proportions of the two reactant monomers and their relative reactivities. In practice, neither perfectly random nor perfectly alternating copolymers are usually found. Most copolymers have many random imperfections.
Two other forms of copolymers that can be prepared under certain conditions are called block copolymers and graft copolymers. Block copolymers are those in which different blocks of identical monomer units alternate with each other; graft copolymers are those in which homopolymer branches of one monomer unit are “grafted” onto a homopolymer chain of another monomer unit.
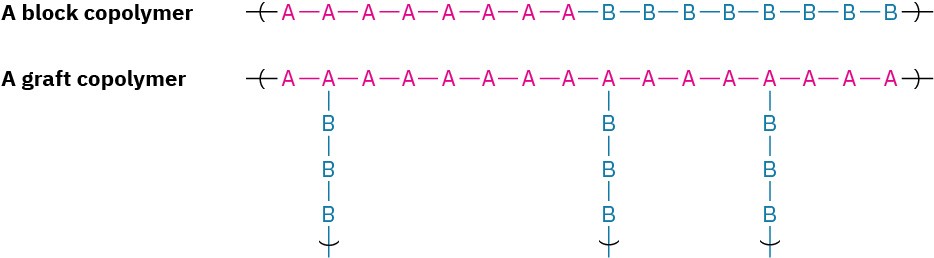
Block copolymers are prepared by initiating the polymerization of one monomer as if growing a homopolymer chain and then adding an excess of the second monomer to the still-active reaction mix. Graft copolymers are made by gamma irradiation of a completed homopolymer chain in the presence of the second monomer. The high-energy irradiation knocks hydrogen atoms off the homopolymer chain at random points, thus generating new radical sites that can initiate polymerization of the added monomer.
Problem 31-6
Draw the structure of an alternating segment of butyl rubber, a copolymer of isoprene (2- methyl-1,3-butadiene) and isobutylene (2-methylpropene) prepared using a cationic initiator.
Problem 31-7
Irradiation of poly(1,3-butadiene), followed by addition of styrene, yields a graft copolymer that is used to make rubber soles for shoes. Draw the structure of a representative segment of this styrene–butadiene graft copolymer.