When heated under either acidic or basic conditions, the β-hydroxy aldehydes or ketones formed in aldol reactions can be dehydrated to yield α,β-unsaturated products, or conjugated enones (ene + ketone). In fact, it’s this loss of water that gives the carbonyl condensation reaction its name, because water condenses out when an enone product forms.
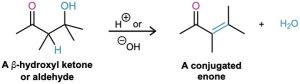
Most alcohols are resistant to dehydration by base (Section 17.6) because hydroxide ion is a poor leaving group, but aldol products dehydrate easily because of their carbonyl group. Under basic conditions, an acidic α hydrogen is removed, yielding an enolate ion that expels the nearby –OH leaving group in an E1cB reaction (Section 11.10). Under acidic conditions, an enol is formed, the –OH group is protonated, and water is expelled in an E1 or E2 reaction.
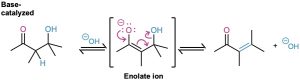
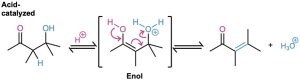
The reaction conditions needed for aldol dehydration are often only a bit more vigorous (slightly higher temperature, for instance) than the conditions needed for the aldol formation itself. As a result, conjugated enones are usually obtained directly from aldol reactions without isolating the intermediate β-hydroxy carbonyl compounds.
Conjugated enones are more stable than nonconjugated enones for the same reason that conjugated dienes are more stable than nonconjugated dienes (Section 14.1). Interaction between the π electrons of the C═C bond and the π electrons of the C═O group leads to a molecular-orbital description for a conjugated enone with an interaction of the π electrons over all four atomic centers (Figure 23.3).
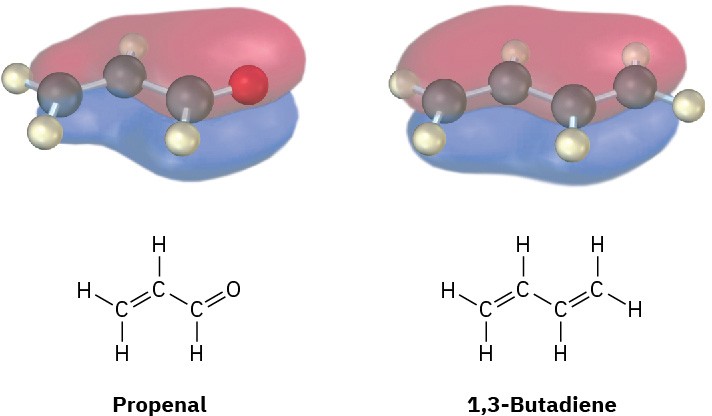 Figure 23.3 The π bonding molecular orbitals of a conjugated enone (propenal) and a conjugated diene (1,3-butadiene) are similar in shape and are spread over the entire π system.
Figure 23.3 The π bonding molecular orbitals of a conjugated enone (propenal) and a conjugated diene (1,3-butadiene) are similar in shape and are spread over the entire π system.
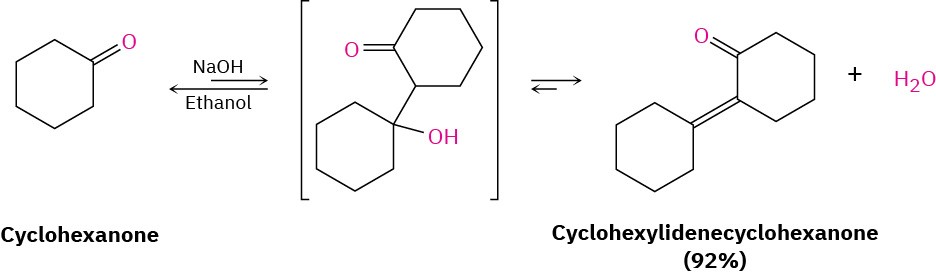
The real value of aldol dehydration is that removal of water from the reaction mixture can be used to drive the aldol equilibrium toward the product. Even though the initial aldol step itself may be unfavorable, as it usually is for ketones, the subsequent dehydration step nevertheless allows many aldol condensations to be carried out in good yield.
Cyclohexanone, for example, gives cyclohexylidenecyclohexanone in 92% yield, even though the initial equilibrium is unfavorable.
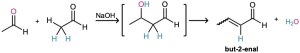
Worked Example 23.2 – Predicting the Product of an Aldol Reaction
What is the structure of the enone obtained from aldol condensation of acetaldehyde?
Strategy: In the aldol reaction, H2O is eliminated and a double bond is formed by removing two hydrogens from the acidic α position of one partner and the carbonyl oxygen from the second partner. The product is thus an α,β-unsaturated aldehyde or ketone.
Solution:
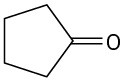
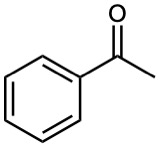
Problem 23-3
What enone product would you expect from aldol condensation of each of the following compounds?
(a)
(b)
(c)
![]()
Problem 23-4
Aldol condensation of 3-methylcyclohexanone leads to a mixture of two enone products, not counting double-bond isomers. Draw them.

