Glyceraldehyde, the simplest aldose, has only one chirality center and thus has two enantiomeric (nonidentical mirror-image) forms. Only the dextrorotatory enantiomer occurs naturally, however. That is, a sample of naturally occurring glyceraldehyde placed in a polarimeter rotates plane-polarized light in a clockwise direction, denoted (+). Since (+)-glyceraldehyde has been found to have an R configuration at C2, it can be represented by a Fischer projection as shown previously in Figure 25.2. For historical reasons dating back long before the adoption of the R,S system, (R)-(+)-glyceraldehyde is also referred to as D-glyceraldehyde (D for dextrorotatory). The other enantiomer, (S)-(–)-glyceraldehyde, is known as L-glyceraldehyde (L for levorotatory).
Because of the way that monosaccharides are biosynthesized in nature, glucose, fructose, and most other naturally occurring monosaccharides all have the same R stereochemical configuration as D-glyceraldehyde at the chirality center farthest from the carbonyl group. In Fischer projections, therefore, most naturally occurring sugars have the hydroxyl group at the bottom chirality center pointing to the right (Figure 25.3). Such compounds are referred to as D sugars.
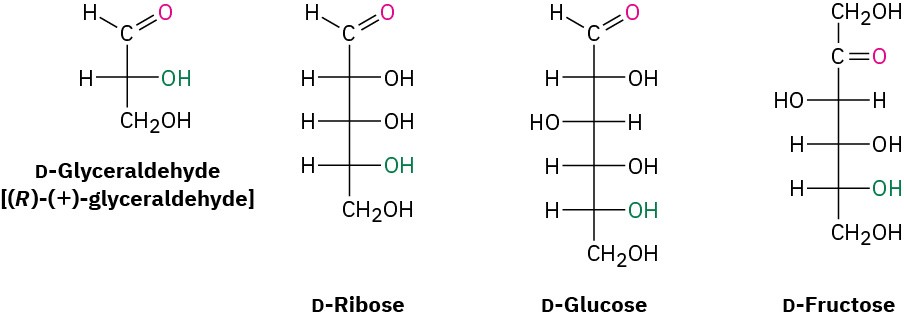
Figure 25.3 Some naturally occurring D sugars. The –OH group at the chirality center farthest from the carbonyl group has the same configuration as (R)-(+)-glyceraldehyde and points toward the right in Fischer projections.
In contrast with D sugars, L sugars have an S configuration at the lowest chirality center, with the bottom –OH group pointing to the left in Fischer projections. Thus, an L sugar is the mirror image (enantiomer) of the corresponding D sugar and has the opposite configuration from the D sugar at all chirality centers.
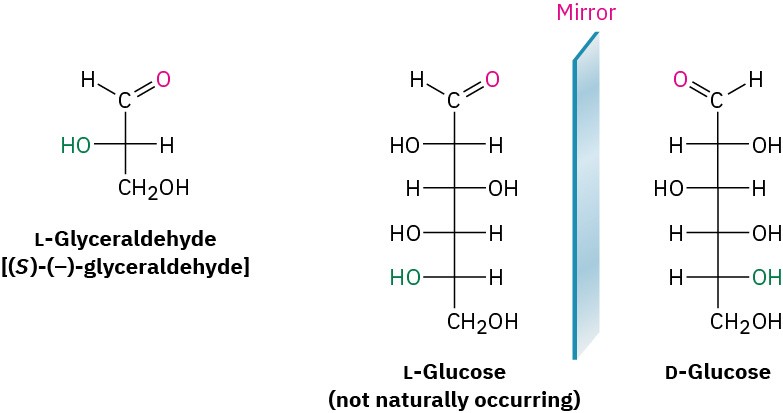
Note that the D and L notations have no relation to the direction in which a given sugar rotates plane-polarized light. A D sugar can be either dextrorotatory or levorotatory. The prefix D indicates only that the –OH group at the lowest chirality center has R stereochemistry and points to the right when the molecule is drawn in a standard Fischer projection. Note also that the D,L system of carbohydrate nomenclature describes the configuration at only one chirality center and says nothing about the configuration of other chirality centers that may be present.
Problem 25-6
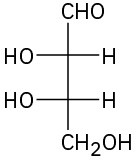
Assign R or S configuration to each chirality center in the following monosaccharides, and tell whether each is a D sugar or an L sugar:
(a)
(b)
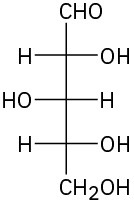
(c)
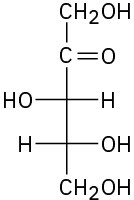
Problem 25-7
(+)-Arabinose, an aldopentose that is widely distributed in plants, is systematically named (2R,3S,4S)-2,3,4,5-tetrahydroxypentanal. Draw a Fischer projection of (+)-arabinose, and identify it as a D sugar or an L sugar.

