Let’s cover just one more point before ending this introductory chapter. In the structures we’ve been drawing until now, a line between atoms has represented the two electrons in a covalent bond. Drawing every bond and every atom is tedious, however, so chemists have devised several shorthand ways for writing structures. In condensed structures, C–H and C–C single bonds aren’t shown; instead, they’re understood. If a carbon has three hydrogens bonded to it, we write CH3; if a carbon has two hydrogens bonded to it, we write CH2; and so on. The compound called 2-methylbutane, for example, is written as follows:
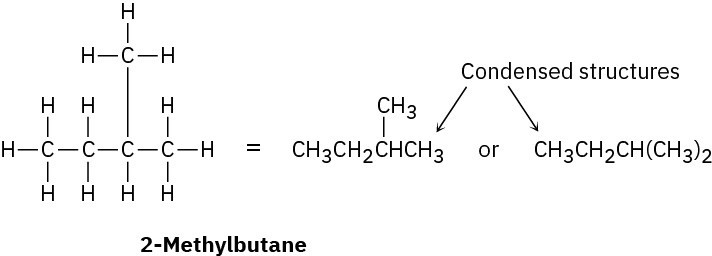
Note that the horizontal bonds between carbons aren’t shown in condensed structures— the CH3, CH2, and CH units are simply placed next to each other—but vertical C–C bonds like that of the first of the condensed structures drawn above is shown for clarity. Notice also in the second of the condensed structures that the two CH3 units attached to the CH carbon are grouped together as (CH3)2.
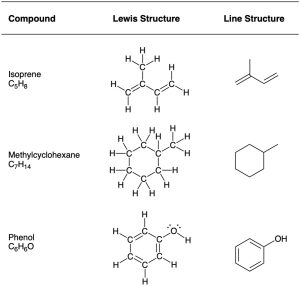
Even simpler than condensed structures are line structures such as those shown in Table 1.3. The rules for drawing line structures are straightforward.
RULE 1
Carbon atoms aren’t usually shown. Instead, a carbon atom is assumed to be at each intersection of two lines (bonds) and at the end of each line. Occasionally, a carbon atom might be indicated for emphasis or clarity.
RULE 2
Hydrogen atoms bonded to carbon aren’t shown. Because carbon has a valence of 4, we mentally supply the correct number of hydrogen atoms for each carbon.
RULE 3
Atoms other than carbon and hydrogen are shown.
One further comment: Although such groupings as –CH3, –OH, and –NH2 are usually written with the C, O, or N atom first and the H atom second, the order of writing is sometimes inverted to H3C–, HO–, and H2N– if needed to make the bonding connections clearer. Larger units such as –CH2CH3 are not inverted, though; we don’t write H3CH2C– because it would be confusing. There are, however, no well-defined rules that cover all cases; it’s largely a matter of preference.
Table 1.3 Lewis and Line Structures for Some Compounds
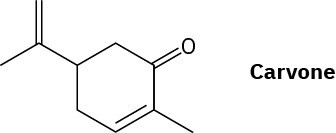
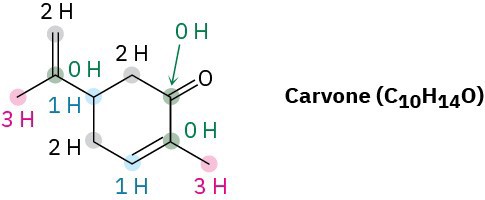
Worked Example 1.4 Interpreting a Line Structure – Carvone, a substance responsible for the odor of spearmint, has the following structure. Tell how many hydrogens are bonded to each carbon, and give the molecular formula of carvone.
Strategy: The end of a line represents a carbon atom with 3 hydrogens, CH3; a two-way intersection is a carbon atom with 2 hydrogens, CH2; a three-way intersection is a carbon atom with 1 hydrogen, CH; and a four-way intersection is a carbon atom with no attached hydrogens.
Solution
Problem 1-15
How many hydrogens are bonded to each carbon in the following compounds, and what is the molecular formula of each substance?
(a)
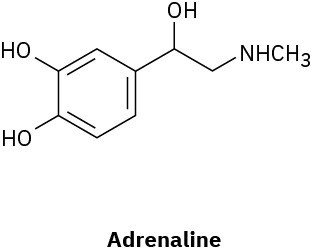
(b)
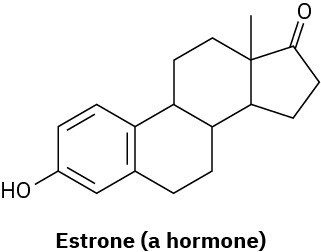
Problem 1-16
Propose line structures for compounds that satisfy the following molecular formulas: There is more than one possibility in each case.
(a) C5H12 (b) C2H7N (c) C3H6O (d) C4H9Cl
Problem 1-17
The following molecular model is a representation of para-aminobenzoic acid (PABA), the active ingredient in many sunscreens. Indicate the positions of the multiple bonds, and draw a line structure (black = C, red = O, blue = N, gray = H).
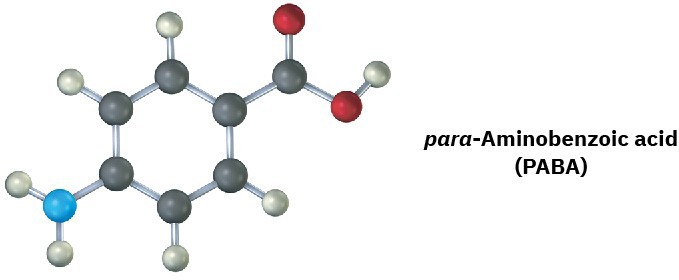
Additional Problems 1
Visualizing Chemistry
Problem 1-18
Convert each of the following molecular models into a line structure, and give the formula of each. Only the connections between atoms are shown; multiple bonds are not indicated (black = C, red = O, blue = N, gray = H).
(a)
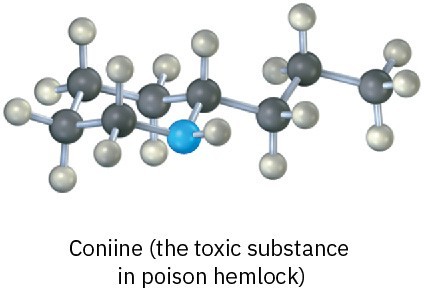
(b)
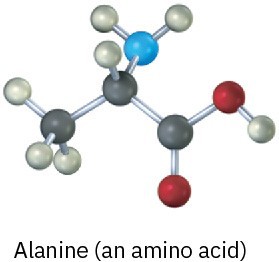
Problem 1-19
The following model is a representation of citric acid, the key substance in the so-called citric acid cycle, by which food molecules are metabolized in the body. Only the connections between atoms are shown; multiple bonds are not indicated. Complete the structure by indicating the positions of multiple bonds and lone-pair electrons (black = C, red = O, gray = H).
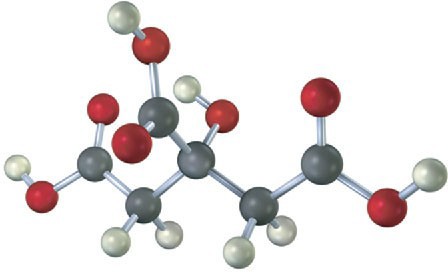
Problem 1-20
The following model is a representation of acetaminophen, a pain reliever sold in drugstores under a variety of names, including Tylenol. Identify the hybridization of each carbon atom in acetaminophen, and tell which atoms have lone pairs of electrons (black = C, red = O, blue = N, gray = H).
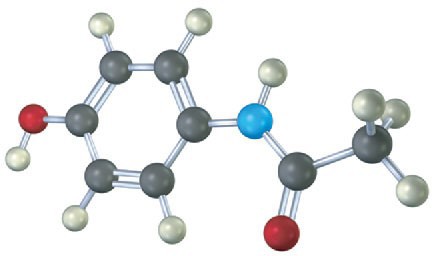
Problem 1-21
The following model is a representation of aspartame, C14H18N2O5, known commercially under many names, including NutraSweet. Only the connections between atoms are shown; multiple bonds are not indicated. Complete the structure for aspartame, and indicate the positions of multiple bonds (black = C, red = O, blue = N, gray = H).
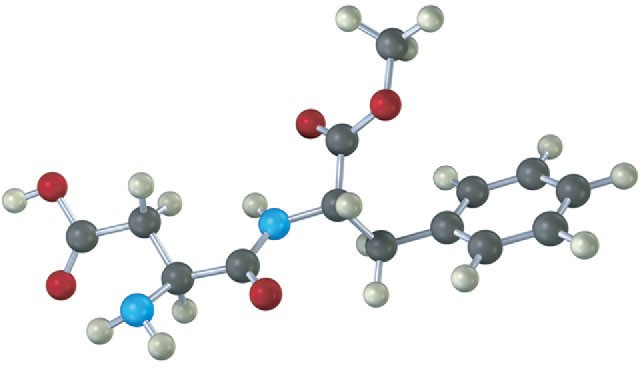
Electron Configurations
Problem 1-22
How many valence electrons does each of the following dietary trace elements have?
(a) Zinc (b) Iodine (c) Silicon (d) Iron
Problem 1-23
Give the ground-state electron configuration for each of the following elements:
(a) Potassium (b) Arsenic (c) Aluminum (d) Germanium
Lewis and Line Structures
Problem 1-24
What are likely formulas for the following molecules?
(a) NH?OH (b) AlCl? (c) CF2Cl? (d) CH?O
Problem 1-25
Why can’t molecules with the following formulas exist?
(a) CH5 (b) C2H6N (c) C3H5Br2
Problem 1-26
Draw a Lewis structure for acetonitrile, C2H3N, which contains a carbon–nitrogen triple bond. How many electrons does the nitrogen atom have in its outer shell? How many are bonding, and how many are nonbonding?
Problem 1-27
Draw a line structure for vinyl chloride, C2H3Cl, the starting material from which PVC poly(vinyl chloride) plastic is made.
Problem 1-28
Fill in any lone pair electrons that are missing from the following structures:
(a)
(b)
(c)
Problem 1-29
Convert the following Lewis structures into molecular formulas:
(a)
(b)
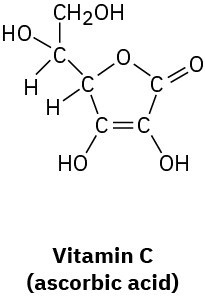
(c)
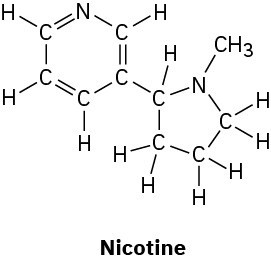
(d)
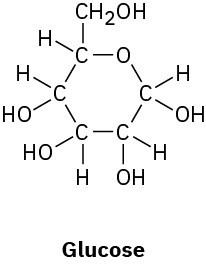
Problem 1-30
Convert the following molecular formulas into line structures that are consistent with valence rules:
(a) C3H8
(b) CH5N
(c) C2H6O (2 possibilities)
(d) C3H7Br (2 possibilities)
(e) C2H4O (3 possibilities)
(f) C3H9N (4 possibilities)
Problem 1-31
Draw a three-dimensional representation of the oxygen-bearing carbon atom in ethanol, CH3CH2OH, using the standard convention of solid, wedged, and dashed lines.
Problem 1-32
Oxaloacetic acid, an important intermediate in food metabolism, has the formula C4H4O5 and contains three C=O bonds and two O–H bonds. Propose two possible structures.
Problem 1-33
Draw structures for the following molecules, showing lone pairs:
(a) Acrylonitrile, C3H3N, which contains a carbon–carbon double bond and a carbon–nitrogen triple bond
(b) Ethyl methyl ether, C3H8O, which contains an oxygen atom bonded to two carbons
(c) Butane, C4H10, which contains a chain of four carbon atoms
(d) Cyclohexene, C6H10, which contains a ring of six carbon atoms and one carbon–carbon double bond
Hybridization
Problem 1-34
What is the hybridization of each carbon atom in acetonitrile (Problem 1-26)?
Problem 1-35
What kind of hybridization do you expect for each carbon atom in the following molecules?
(a) ![]()
(b) ![]()
(c) ![]()
(d) 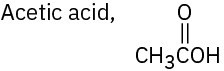
Problem 1-36
What is the shape of benzene, and what hybridization do you expect for each carbon?
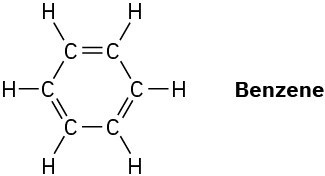
Problem 1-37
What bond angle do you expect for each of the indicated atoms, and what kind of hybridization do you expect for the central atom in each molecule?
(a)
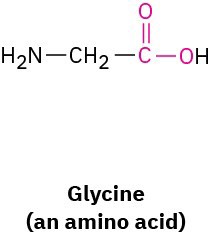
(b)
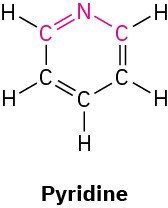
(c)
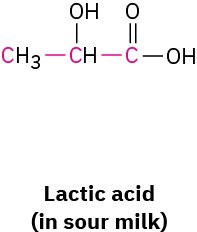
Problem 1-38
Propose structures for molecules that meet the following descriptions:
(a) Contains two sp2-hybridized carbons and two sp3-hybridized carbons
(b) Contains only four carbons, all of which are sp2-hybridized
(c) Contains two sp-hybridized carbons and two sp2-hybridized carbons
Problem 1-39
What kind of hybridization do you expect for each carbon atom in the following molecules:
(a)
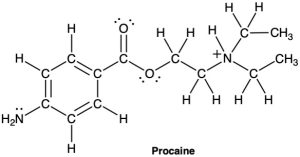
(b)
Problem 1-40
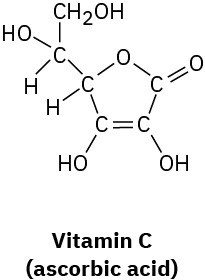
Pyridoxal phosphate, a close relative of vitamin B6, is involved in a large number of metabolic reactions. What is the hybridization and the bond angle for each nonterminal atom?
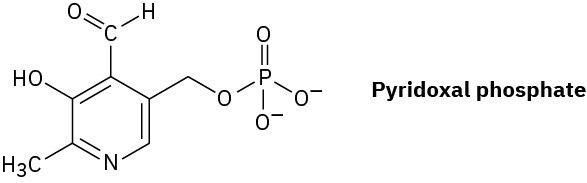
Line Structures
Problem 1-41
Convert the following Lewis structures into line structures:
(a)
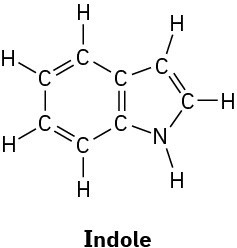
(b)
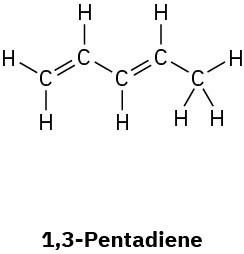
(c)
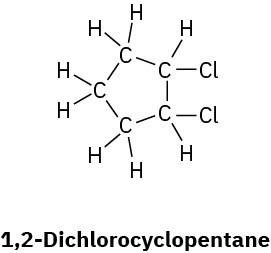
(d)
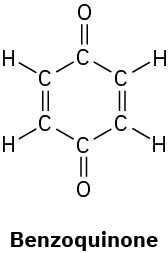
Problem 1-42
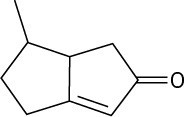
How many hydrogens are bonded to each carbon atom in the following substances, and what is the molecular formula of each?
(a)
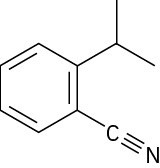
(b)
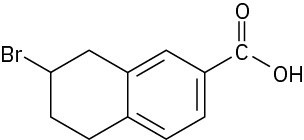
(c)
Problem 1-43
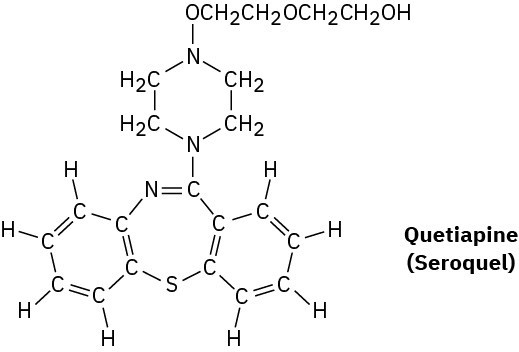
Quetiapine, marketed as Seroquel, is a heavily prescribed antipsychotic drug used in the treatment of schizophrenia and bipolar disorder. Convert the following representation into a line structure, and give the molecular formula of quetiapine.
Problem 1-44
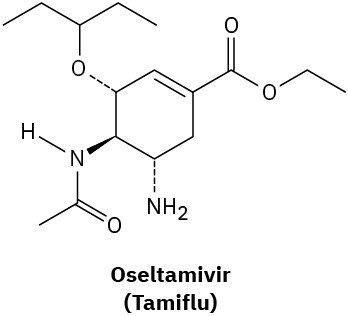
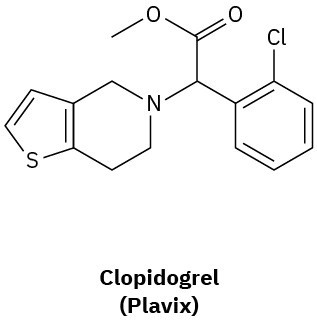
How many hydrogens are bonded to each carbon atom in (a) the antiinfluenza agent oseltamivir, marketed as Tamiflu, and (b) the platelet aggregation inhibitor clopidogrel, marketed as Plavix? Give the molecular formula of each.
(a)
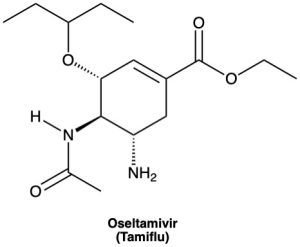
(b)
General Problems
Problem 1-45
Why do you suppose no one has ever been able to make cyclopentyne as a stable molecule?

Problem 1-46
Allene, H2C=C=CH2, has two adjacent double bonds. Draw a picture showing the orbitals involved in the σ and π bonds of allene. Is the central carbon atom sp2– or sp-hybridized? What about the hybridization of the terminal carbons? What shape do you predict for allene?
Problem 1-47
Allene (see Problem 1-46) is structurally related to carbon dioxide, CO2. Draw a picture showing the orbitals involved in the σ and π bonds of CO2, and identify the likely hybridization of carbon.
Problem 1-48
Complete the electron-dot structure of caffeine, showing all lone-pair electrons, and identify the hybridization of the indicated atoms.
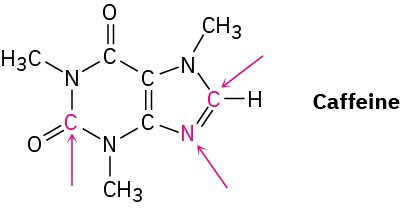
Problem 1-49
Most stable organic species have tetravalent carbon atoms, but species with trivalent carbon atoms also exist. Carbocations are one such class of compounds.

(a) How many valence electrons does the positively charged carbon atom have?
(b) What hybridization do you expect this carbon atom to have?
(c) What geometry is the carbocation likely to have?
Problem 1-50
A carbanion is a species that contains a negatively charged, trivalent carbon.

(a) What is the electronic relationship between a carbanion and a trivalent nitrogen compound such as NH3?
(b) How many valence electrons does the negatively charged carbon atom have?
(c) What hybridization do you expect this carbon atom to have?
(d) What geometry is the carbanion likely to have?
Problem 1-51
Divalent carbon species called carbenes are capable of fleeting existence. For example, methylene, :CH2, is the simplest carbene. The two unshared electrons in methylene can be either paired in a single orbital or unpaired in different orbitals. Predict the type of hybridization you expect carbon to adopt in singlet (spin-paired) methylene and triplet (spin-unpaired) methylene. Draw a picture of each, and identify the valence orbitals on carbon.
Problem 1-52
Two different substances have the formula C4H10. Draw both, and tell how they differ.
Problem 1-53
Two different substances have the formula C3H6. Draw both, and tell how they differ.
Problem 1-54
Two different substances have the formula C2H6O. Draw both, and tell how they differ.
Problem 1-55
Three different substances contain a carbon–carbon double bond and have the formula C4H8. Draw them, and tell how they differ.
Problem 1-56
Among the most common over-the-counter drugs you might find in a medicine cabinet are mild pain relievers such ibuprofen (Advil, Motrin), naproxen (Aleve), and acetaminophen (Tylenol).
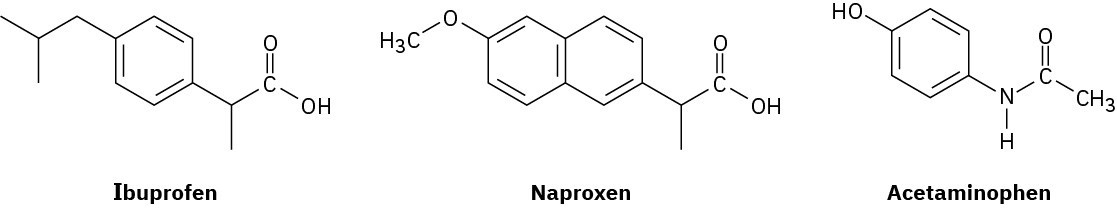
(a) How many sp3-hybridized carbons does each molecule have?
(b) How many sp2-hybridized carbons does each molecule have?
(c) What similarities can you see in their structures?

