An enzyme is a substance—usually a large protein—that acts as a catalyst for a biological reaction. Like all catalysts, an enzyme doesn’t affect the equilibrium constant of a reaction and can’t bring about a chemical change that is otherwise unfavorable. An enzyme acts only to lower the activation energy for a reaction, thereby making the reaction take place more rapidly than it otherwise would. Sometimes, in fact, the rate acceleration brought about by enzymes is extraordinary. Millionfold rate increases are common, and the glycosidase enzymes that hydrolyze polysaccharides increase the reaction rate by a factor of more than 1017, changing the time required for the reaction from millions of years to milliseconds!
Unlike many of the catalysts that chemists use in the laboratory, enzymes are usually specific in their action. Often, in fact, an enzyme will catalyze only a single reaction of a single compound, called the enzyme’s substrate. For example, the enzyme amylase, found in the human digestive tract, catalyzes only the hydrolysis of starch to yield glucose; cellulose and other polysaccharides are untouched by amylase.
Different enzymes have different specificities. Some, such as amylase, are specific for a single substrate, but others operate on a range of substrates. Papain, for instance, a globular protein of 212 amino acids isolated from papaya fruit, catalyzes the hydrolysis of many kinds of peptide bonds. In fact, it’s this ability to hydrolyze peptide bonds that makes papain useful as a cleaner for contact lenses.
Enzymes function through a pathway that involves initial formation of an enzyme– substrate complex (E · S), followed by a multistep chemical conversion of the enzyme- bound substrate into enzyme-bound product (E · P) and final release of product from the complex.
E + S ⇄ E ⋅ S ⇄ E ⋅ P ⇄ E + P
The overall rate constant for conversion of the E · S complex to products E · P is called the turnover number because it represents the number of substrate molecules a single enzyme molecule turns over into product per unit time. A value of about 103 per second is typical, although carbonic anhydrase can reach a value of up to 600,000.
The extraordinary rate accelerations achieved by enzymes are due to a combination of several factors. One important factor is simple geometry: an enzyme will adjust its shape to hold the substrate, other reactants, and various catalytic sites on acidic or basic residues in the precise geometry needed for reaction. In addition, the wrapping of the enzyme around the substrate can create specialized microenvironments that protect the substrate from the aqueous medium and can dramatically change the behavior of acid–base catalytic residues in the active site. But perhaps most important is that the enzyme stabilizes and thus lowers
the energy of the rate-limiting transition state for reaction. That is, it’s not the ability of the enzyme to bind the substrate that matters but rather its ability to bind and stabilize the transition state. Often, in fact, the enzyme binds the transition structure as much as 1012 times more tightly than it binds the substrate or products. An energy diagram for an enzyme-catalyzed process might resemble that in Figure 26.9.
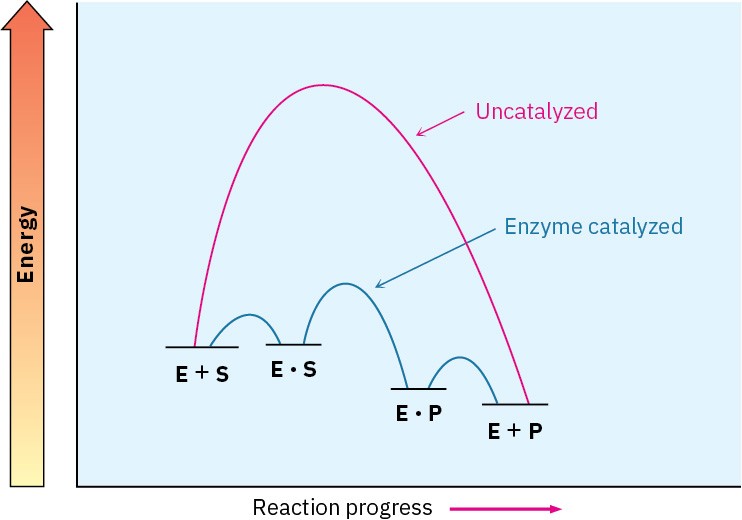
Figure 26.9 Energy diagrams for uncatalyzed and enzyme-catalyzed processes. The enzyme makes available an alternative, lower-energy pathway. Rate enhancement is due to the ability of the enzyme to bind to the transition state for product formation, thereby lowering its energy.
Enzymes are classified into six categories depending on the kind of reaction they catalyze, as shown in Table 26.2. Oxidoreductases catalyze oxidations and reductions; transferases catalyze the transfer of a group from one substrate to another; hydrolases catalyze hydrolysis reactions of esters, amides, and related substrates; lyases catalyze the elimination or addition of a small molecule such as H2O from or to a substrate; isomerases catalyze isomerizations; and ligases catalyze the bonding of two molecules, often coupled with the hydrolysis of ATP. The systematic name of an enzyme has two parts, ending with – ase. The first part identifies the enzyme’s substrate, and the second part identifies its class. Hexose kinase, for example, is a transferase that catalyzes the transfer of a phosphate group from ATP to a hexose sugar.
Table 26.2 Classification of Enzymes
|
Class |
Some subclasses |
Function |
|
Oxidoreductases |
Dehydrogenases Oxidases Reductases |
Introduction of double bond Oxidation Reduction |
|
Transferases |
Kinases |
Transfer of phosphate group |
|
Some subclasses |
Function |
|
|
|
Transaminases |
Transfer of amino group |
|
Hydrolases |
Lipases Nucleases Proteases |
Hydrolysis of ester Hydrolysis of phosphate Hydrolysis of amide |
|
Lyases |
Decarboxylases Dehydrases |
Loss of CO2 Loss of H2O |
|
Isomerases |
Epimerases |
Isomerization of chirality center |
|
Ligases |
Carboxylases Synthetases |
Addition of CO2 Formation of a new bond |
In addition to their protein part, most enzymes also contain a small non-protein part called a cofactor. A cofactor can be either an inorganic ion, such as Zn2+, or a small organic molecule, called a coenzyme. A coenzyme is not a catalyst but is a reactant that undergoes chemical change during the reaction and requires an additional step to return to its initial state.
Many coenzymes are derived from vitamins—substances that an organism requires in small amounts for growth but is unable to synthesize and must receive in its diet (Chapter 20 Chemistry Matters). Coenzyme A from pantothenate (vitamin B3), NAD+ from niacin, FAD from riboflavin (vitamin B2), tetrahydrofolate from folic acid, pyridoxal phosphate from pyridoxine (vitamin B6), and thiamin diphosphate from thiamin (vitamin B1) are examples. Table 26.3 shows the structures of some common coenzymes.
Problem 26-18
To what classes do the following enzymes belong? (a)
Pyruvate decarboxylase (b)
Chymotrypsin (c)
Alcohol dehydrogenase

