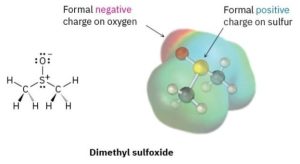
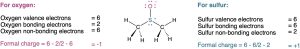
Closely related to the ideas of bond polarity and dipole moment is the assignment of formal charges to specific atoms within a molecule, particularly atoms that have an apparent “abnormal” number of bonds. Consider the below representation of dimethyl sulfoxide (CH3SOCH3), a solvent commonly used for preserving biological cell lines at low temperature. The sulfur atom in dimethyl sulfoxide has three bonds (rather than the usual two) and has a formal positive charge. The oxygen atom, by contrast, has one bond (rather than the usual two) and has a formal negative charge. Note that an electrostatic potential map of dimethyl sulfoxide shows the oxygen as electron rich (red) and the sulfur as electron poor (blue), in accordance with these formal charges.
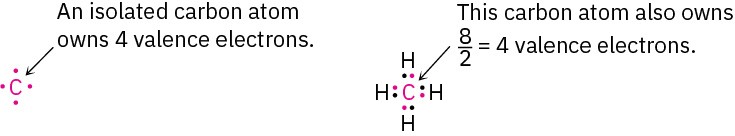
Formal charges, as the name suggests, are a formalism and do not imply the presence of actual ionic charges in a molecule. Instead, they’re a device for electron “bookkeeping” and can be thought of in the following way: A typical covalent bond is formed when each atom donates one electron. Although the bonding electrons are shared by both atoms, each atom can still be considered to “own” one electron for bookkeeping purposes. In methane, for instance, the carbon atom “owns” one electron in each of the four C–H bonds. Because a neutral, isolated carbon atom has four valence electrons, and because the carbon atom in methane still owns four, the methane carbon atom is neutral and has no formal charge.
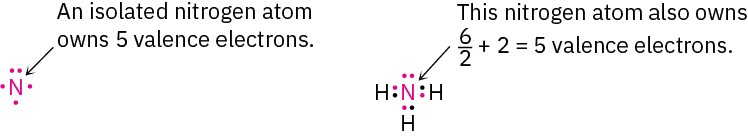
The same is true for the nitrogen atom in ammonia, which has three covalent N–H bonds and two nonbonding electrons (a lone pair). Atomic nitrogen has five valence electrons, and the ammonia nitrogen also has five—one in each of three shared N–H bonds plus two in the lone pair. Thus, the nitrogen atom in ammonia has no formal charge.
The situation is different in dimethyl sulfoxide. Atomic sulfur has six valence electrons, but the dimethyl sulfoxide sulfur “owns” only five—one in each of the two S–C single bonds, one in the S–O single bond, and two in a lone pair. Thus, the sulfur atom has formally lost an electron and therefore has a positive formal charge. A similar calculation for the oxygen atom shows that it has formally gained an electron and has a negative charge. Atomic oxygen has six valence electrons, but the oxygen in dimethyl sulfoxide has seven—one in the O–S bond and two in each of three lone pairs. Thus, the oxygen has formally gained an electron and has a negative formal charge.
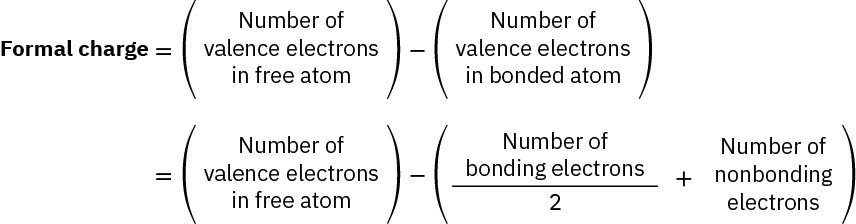
To express the calculations in a general way, the formal charge on an atom is equal to the number of valence electrons in a neutral, isolated atom minus the number of electrons “owned” by that bonded atom in a molecule. The number of electrons “owned” by the bonded atom is equal to half the number of bonding electrons plus all of the nonbonding electrons.
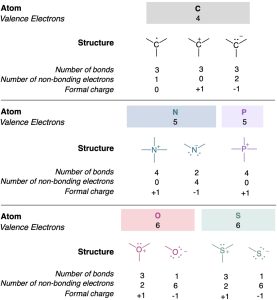
A summary of commonly encountered formal charges and the bonding situations in which they occur is given in Table 2.2. Although only a bookkeeping device, formal charges often give clues about chemical reactivity, so it’s helpful to be able to identify and calculate them correctly.
Table 2.2 A Summary of Common Formal Charges
Problem 2-7 Calculate formal charges for the nonhydrogen atoms in the following molecules:
(a) ![]()
(b) ![]()
(c) ![]()
Problem 2-8
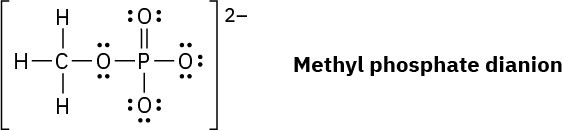
Organic phosphate groups occur commonly in biological molecules. Calculate formal charges on the four O atoms in the methyl phosphate dianion.