The structural features that make it possible to classify compounds into families are called functional groups. A functional group is a group of atoms within a molecule that has a characteristic chemical behavior. Chemically, a given functional group behaves in nearly the same way in every molecule it’s a part of. For example, compare ethylene, a plant hormone that causes fruit to ripen, with menthene, a much more complicated molecule found in peppermint oil. Both substances contain a carbon–carbon double-bond functional group, and both therefore react with Br2 in the same way to give a product in which a Br atom has added to each of the double-bond carbons (Figure 3.2). This example is typical: the chemistry of every organic molecule, regardless of size and complexity, is determined by the functional groups it contains.
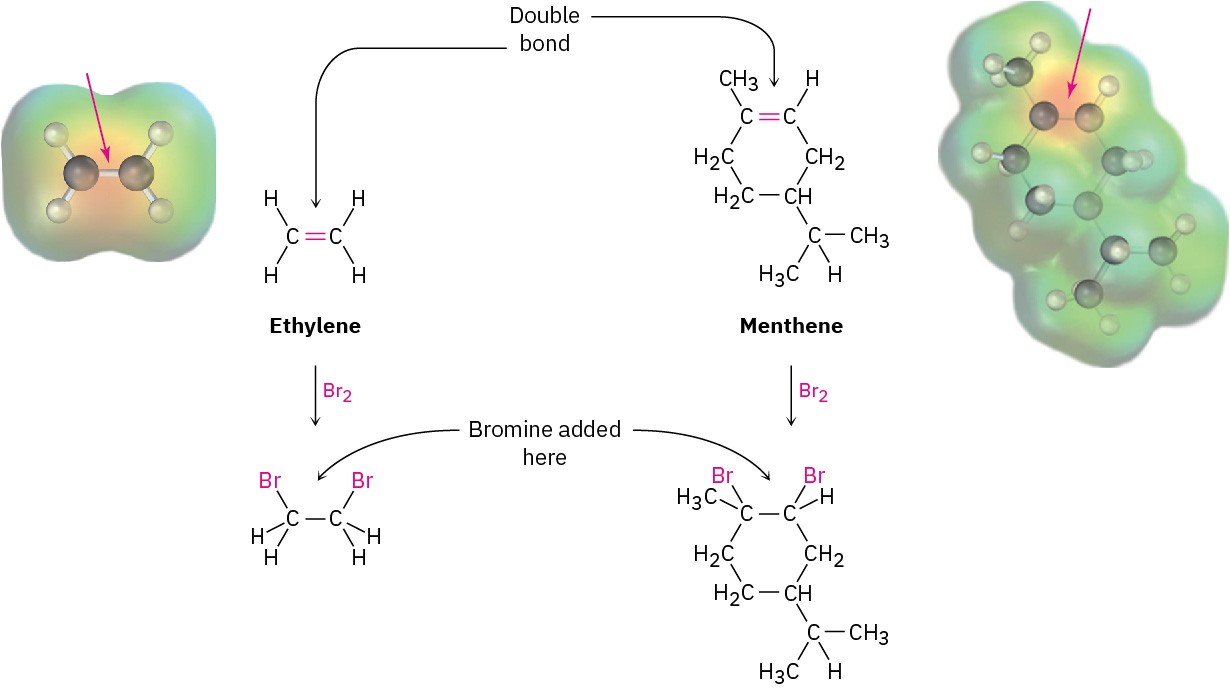
Figure 3.2 The reactions of ethylene and menthene with bromine. In both molecules, the carbon–carbon double-bond functional group react with Br2 in the same way. The size and complexity of the molecules are not important.
Look at Table 3.1, which lists many of the common functional groups and gives simple examples of their occurrence. Some functional groups have only carbon–carbon double or triple bonds; others have halogen atoms; and still others contain oxygen, nitrogen, or sulfur. Much of the chemistry you’ll be studying is the chemistry of these functional groups.
Functional Groups with Carbon–Carbon Multiple Bonds
Alkenes have a carbon-carbon double bond, alkynes have a carbon-carbon triple bond, and arenes have alternating double and single bonds in a six-membered ring of carbon atoms. They look different, but because of their structural similarities, they also have chemical similarities.
Table 3.1 Structures of Some Common Functional Groups
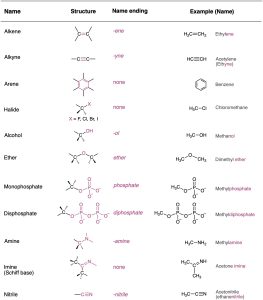
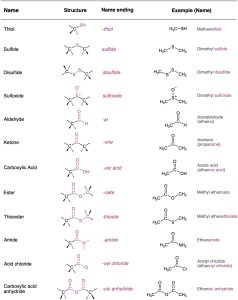
*The bonds whose connections aren’t specified are assumed to be attached to carbon or hydrogen atoms in the rest of the molecule.
Functional Groups with Carbon Singly Bonded to an Electronegative Atom
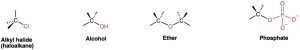
Alkyl halides (haloalkanes), alcohols, ethers, alkyl phosphates, amines, thiols, sulfides, and disulfides all have a carbon atom singly bonded to an electronegative atom—halogen, oxygen, nitrogen, or sulfur. Alkyl halides have a carbon atom bonded to halogen (–X), alcohols have a carbon atom bonded to the oxygen of a hydroxyl group (–OH), ethers have two carbon atoms bonded to the same oxygen, organophosphates have a carbon atom bonded to the oxygen of a phosphate group (–OPO32−), amines have a carbon atom bonded to a nitrogen, thiols have a carbon atom bonded to the sulfur of an –SH group, sulfides have two carbon atoms bonded to the same sulfur, and disulfides have carbon atoms bonded to two sulfurs that are joined together. In all cases, the bonds are polar, with the carbon atom bearing a partial positive charge (δ+) and the electronegative atom bearing a partial negative charge (δ–).


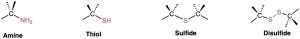
Functional Groups with a Carbon–Oxygen Double Bond (Carbonyl Groups)
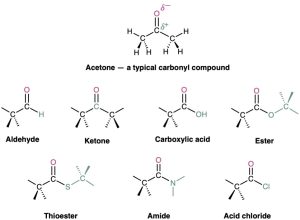
The carbonyl group, C=O (pronounced car–bo–neel) is common to many of the families listed in Table 3.1. Carbonyl groups are present in a majority of organic compounds and in practically all biological molecules. These compounds therefore behave similarly in many respects but differ depending on the identity of the other atoms bonded to the carbonyl carbon. Aldehydes have at least one hydrogen bonded to the C=O, ketones have two carbons bonded to the C=O, carboxylic acids have an –OH group bonded to the C=O, esters have an ether-like oxygen bonded to the C=O, thioesters have a sulfide-like sulfur bonded to the C=O, amides have an amine-like nitrogen bonded to the C=O, acid chlorides have a chlorine bonded to the C=O, and so on. In all these functional groups, the carbonyl carbon atom bears a partial positive charge (δ+), and the oxygen bears a partial negative charge (δ–).
Problem 3-1
Use Table 3.1 to identify the functional groups in each of the following molecules: (a)
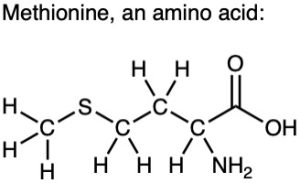
(b)
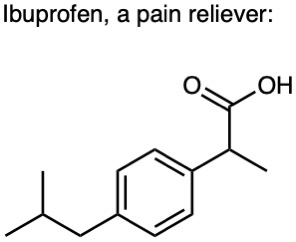
(c)
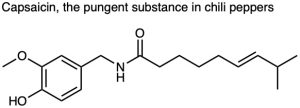
Problem 3-3
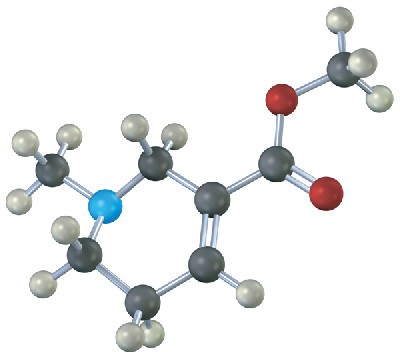
Identify the functional groups in the following model of arecoline, a veterinary drug used to control worms in animals. Convert the drawing into a line-bond structure and a molecular formula (red = O, blue = N, black = C, gray = H).