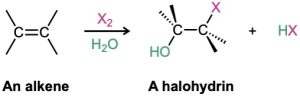
Another example of an electrophilic addition is the reaction an alkene with either Br2 or Cl2 in the presence of water to yield a 1,2-halo alcohol, called a halohydrin.
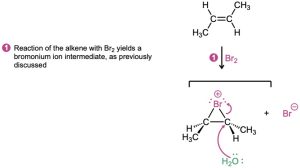
We saw in the previous section that when Br2 reacts with an alkene, the cyclic bromonium ion intermediate reacts with the only nucleophile present, Br− ion. If the reaction is carried out in the presence of an additional nucleophile, however, the intermediate bromonium ion can be intercepted by the added nucleophile and diverted to a different product. In the presence of a high concentration of water, for instance, water competes with Br− ion as a nucleophile and reacts with the bromonium ion intermediate to yield a bromohydrin. The net effect is addition of HO−Br to the alkene by the pathway shown in Figure 8.3.
Figure 8.3 MECHANISM
Bromohydrin formation by reaction of an alkene with Br2 in the presence of water.
Water acts as a nucleophile in step 2 to react with the intermediate bromonium ion.


In practice, few alkenes are soluble in water, and bromohydrin formation is often carried out in a solvent such as aqueous dimethyl sulfoxide, CH3SOCH3 (DMSO), using a reagent called N-bromosuccinimide (NBS) as a source of Br2. NBS is a stable, easily handled compound that slowly decomposes in water to yield Br2 at a controlled rate. Bromine itself can also be used in the addition reaction, but it is more dangerous and more difficult to handle than NBS.
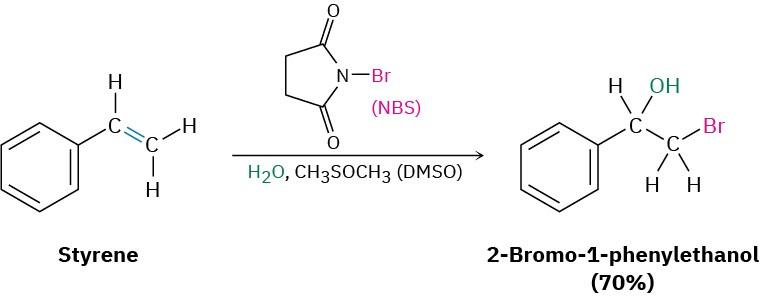
Notice that the aromatic ring in the above example does not react with Br2, even though it appears to have three carbon–carbon double bonds. As we’ll see in Section 15.2, aromatic rings are a good deal more stable and less reactive than might be expected.
There are a number of biological examples of halohydrin formation, particularly in marine organisms. As with halogenation (Section 8.2), halohydrin formation is carried out by haloperoxidases. For example:
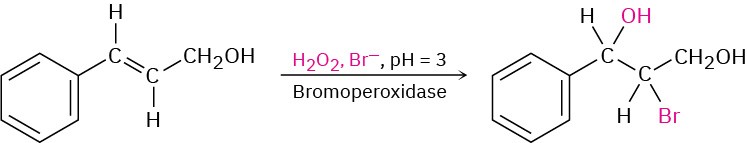
Problem 8-5
What product would you expect from the reaction of cyclopentene with NBS and water? Show the stereochemistry.
Problem 8-6
When an unsymmetrical alkene such as propene is treated with N-bromosuccinimide in aqueous dimethyl sulfoxide, the major product has the bromine atom bonded to the less highly substituted carbon atom. Is this Markovnikov or non-Markovnikov orientation? (Section 7.8) Explain.
![]()

