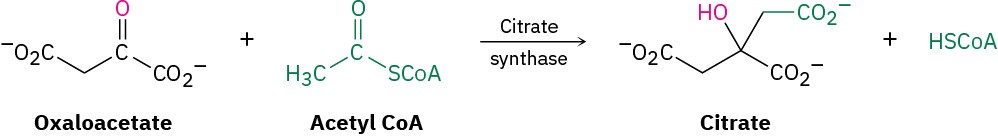
As we saw in the previous section, enzymes work by bringing substrate and other reactant molecules together, holding them in the orientation necessary for reaction, providing any necessary acidic or basic sites to catalyze specific steps, and stabilizing the transition state for reaction. As an example, let’s look at citrate synthase, an enzyme that catalyzes the aldol-like addition of acetyl CoA to oxaloacetate to give citrate. This reaction is the first step in the citric acid cycle, in which acetyl groups produced by degradation of food molecules are metabolized to yield CO2 and H2O. We’ll look at the details of the citric acid cycle in Section 29.7.
Citrate synthase is a globular protein of 433 amino acids with a deep cleft lined by an array of functional groups that can bind to the substrate, oxaloacetate. On binding oxaloacetate, the original cleft closes and another opens up nearby to bind acetyl CoA. This second cleft is also lined by appropriate functional groups, including a histidine at position 274 and an aspartic acid at position 375. The two reactants are now held by the enzyme in close proximity and with a suitable orientation for reaction. Figure 26.10 shows the structure of citrate synthase as determined by X-ray crystallography, along with a close-up of the active site.
Table 26.3 Structures and Functions of Some Common Coenzymes
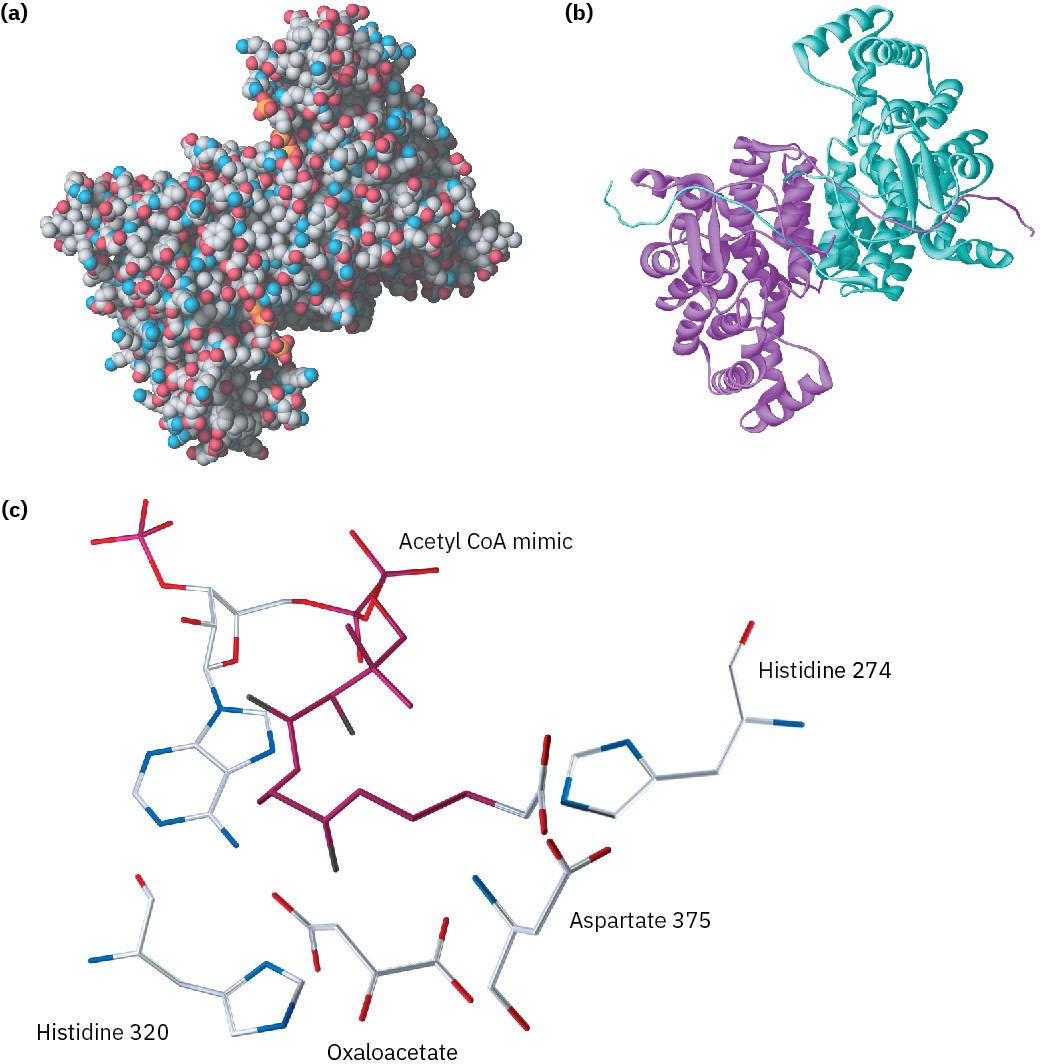
Figure 26.10 X-ray crystal structure of citrate synthase. Part (a) is a space-filling model and part (b) is a ribbon model, which emphasizes the α-helical segments of the protein chain and indicates that the enzyme is dimeric; that is, it consists of two identical chains held together by hydrogen bonds and other intermolecular attractions. Part (c) is a close-up of the active site in which oxaloacetate and an unreactive acetyl CoA mimic are bound.
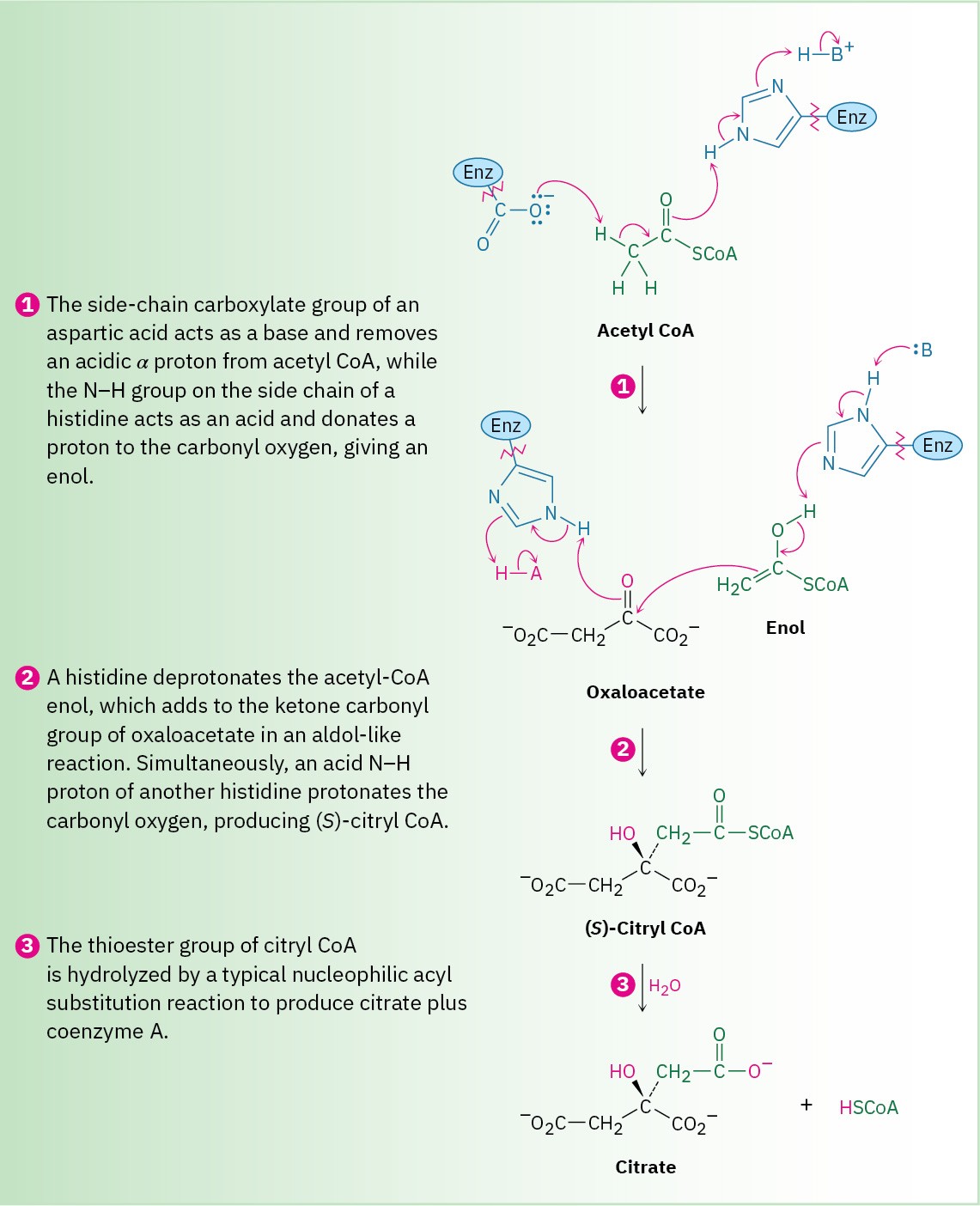
As shown in Figure 26.11, the first step in the aldol reaction of acetyl CoA and oxaloacetate is generation of the enol of acetyl CoA. The side-chain carboxyl of an aspartate residue acts as base to abstract an acidic α proton, while at the same time the side-chain imidazole ring of a histidine donates H+ to the carbonyl oxygen. The enol thus produced then performs a nucleophilic addition to the ketone carbonyl group of oxaloacetate. The first histidine acts as a base to remove the –OH hydrogen from the enol, while a second histidine residue simultaneously donates a proton to the oxaloacetate carbonyl group, giving citryl CoA. Water then hydrolyzes the thiol ester group in citryl CoA in a nucleophilic acyl substitution reaction, releasing citrate and coenzyme A as the final products.
Figure 26.11 MECHANISM
Mechanism of the addition of acetyl CoA to oxaloacetate to give (S)-citryl CoA, catalyzed by citrate synthase.
Additional Problems 26 • Additional Problems 26 • Additional Problems Visualizing Chemistry Problem 26-19
Identify the following amino acids:
(a)
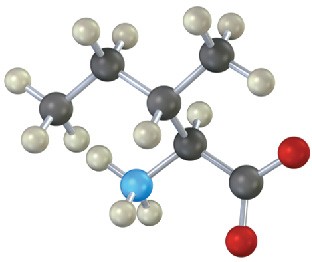
(b)
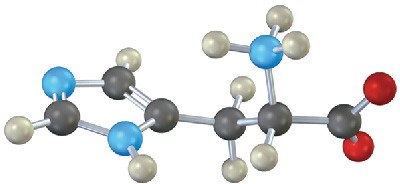
Problem 26-20
Give the sequence of the following tetrapeptide (yellow = S):
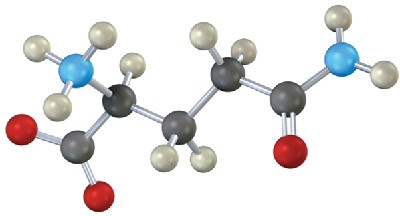
Problem 26-21
Isoleucine and threonine are the only two amino acids with two chirality centers. Assign R
or S configuration to the methyl-bearing carbon atom of isoleucine.
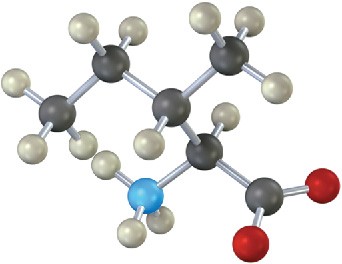
Problem 26-22
Is the following structure a D amino acid or an L amino acid? Identify it.
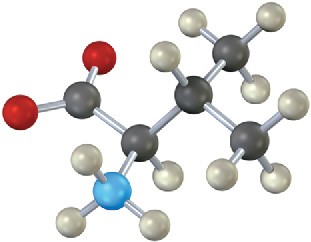
Problem 26-23
Give the sequence of the following tetrapeptide:
Mechanism Problems
Problem 26-24
The reaction of ninhydrin with an α-amino acid occurs in several steps. (a)
The first step is formation of an imine by reaction of the amino acid with ninhydrin. Show its structure and the mechanism of its formation.
(b)
The second step is a decarboxylation. Show the structure of the product and the mechanism of the decarboxylation reaction.
(c)
The third step is hydrolysis of an imine to yield an amine and an aldehyde. Show the structures of both products and the mechanism of the hydrolysis reaction.
The final step is formation of the purple anion. Show the mechanism of the reaction.
Problem 26-25
The chloromethylated polystyrene resin originally used for Merrifield solid-phase peptide synthesis was prepared by treatment of polystyrene with chloromethyl methyl ether and a Lewis acid catalyst. Propose a mechanism for the reaction.
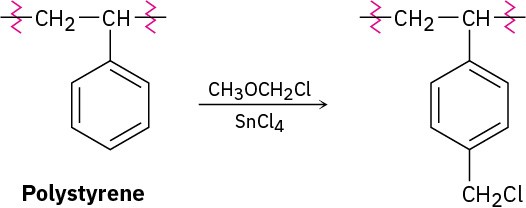
Problem 26-26
An Fmoc protecting group can be removed from an amino acid by treatment with the amine base piperidine. Propose a mechanism.
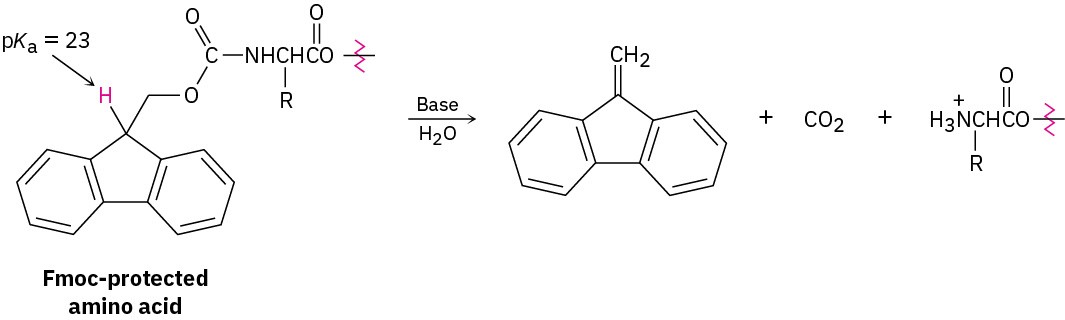
Problem 26-27
Proteins can be cleaved specifically at the amide bond on the carboxyl side of methionine residues by reaction with cyanogen bromide, BrC≡N.
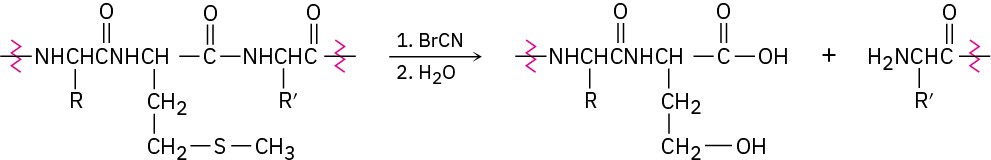
The reaction occurs in several steps:
(a)
The first step is a nucleophilic substitution reaction of the sulfur on the methionine side chain with BrCN to give a cyanosulfonium ion, [R2SCN]+. Show the structure of the product, and propose a mechanism for the reaction.
(b)
The second step is an internal SN2 reaction, with the carbonyl oxygen of the methionine residue displacing the positively charged sulfur leaving group and forming a five- membered ring product. Show the structure of the product and the mechanism of its formation.
(c)
The third step is a hydrolysis reaction to split the peptide chain. The carboxyl group of the former methionine residue is now part of a lactone (cyclic ester) ring. Show the structure of the lactone product and the mechanism of its formation.
(d)
The final step is a hydrolysis of the lactone to give the product shown. Show the mechanism of the reaction.
Problem 26-28
A clever recent method of peptide synthesis involves formation of an amide bond by reaction of an α-keto acid with an N-alkylhydroxylamine:
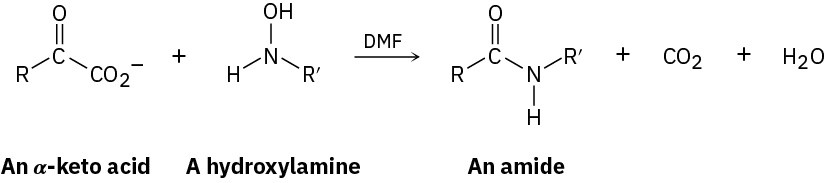
The reaction is thought to occur by nucleophilic addition of the N-alkylhydroxylamine to the keto acid as if forming an oxime (Section 19.8), followed by decarboxylation and elimination of water. Show the mechanism.
Amino Acid Structures and Chirality
Problem 26-29
Except for cysteine, only S amino acids occur in proteins. Several R amino acids are also found in nature, however. (R)-Serine is found in earthworms, and (R)-alanine is found in insect larvae. Draw Fischer projections of (R)-serine and (R)-alanine. Are these D or L amino acids?
Problem 26-30
Cysteine is the only amino acid that has L stereochemistry but an R configuration. Make up a structure for another L amino acid of your own creation that also has an R configuration.
Problem 26-31
Draw a Fischer projection of (S)-proline. Problem 26-32
Show the structures of the following amino acids in their zwitterionic forms: (a)
Trp (b) Ile
(c) Cys (d) His
Problem 26-33
Proline has pKa1 = 1.99 and pKa2 = 10.60. Use the Henderson–Hasselbalch equation to calculate the ratio of protonated and neutral forms at pH = 2.50. Calculate the ratio of neutral and deprotonated forms at pH = 9.70.
Problem 26-34
Using both three- and one-letter codes for amino acids, write the structures of all possible peptides that contain the following amino acids:
(a)
Val, Ser, Leu (b)
Ser, Leu2, Pro Problem 26-35
Look at the side chains of the 20 amino acids in Table 26.1, and then think about what is not present. None of the 20 contain either an aldehyde or a ketone carbonyl group, for instance. Is this just one of nature’s oversights, or is there a likely chemical reason? What complications might an aldehyde or ketone carbonyl group cause?
Amino Acid Synthesis and Reactions
Problem 26-36
Show how you could use the acetamidomalonate method to prepare the following amino acids:
(a) Leucine (b)
Tryptophan Problem 26-37
Show how you could prepare the following amino acids using a reductive amination: (a)
Methionine (b) Isoleucine
Problem 26-38
Show how you could prepare the following amino acids enantioselectively: (a)
Pro (b) Val
Problem 26-39
Serine can be synthesized by a simple variation of the amidomalonate method using formaldehyde rather than an alkyl halide. How might this be done?
Problem 26-40
Predict the product of the reaction of valine with the following reagents: (a)
CH3CH2OH, acid (b)
Di-tert-butyl dicarbonate (c)
KOH, H2O
(d)
CH3COCl, pyridine; then H2O Problem 26-41
Draw resonance forms for the purple anion obtained by reaction of ninhydrin with an α– amino acid (see Problem 26-24).
Peptides and Enzymes
Problem 26-42
Write full structures for the following peptides: (a)
C-H-E-M
(b)
P-E-P-T-I-D-E
Problem 26-43
Propose two structures for a tripeptide that gives Leu, Ala, and Phe on hydrolysis but does not react with phenyl isothiocyanate.
Problem 26-44
Show the steps involved in a synthesis of Phe-Ala-Val using the Merrifield procedure. Problem 26-45
Draw the structure of the PTH derivative product you would obtain by Edman degradation of the following peptides:
(a)
I-L-P-F
(b)
D-T-S-G-A
Problem 26-46
Which amide bonds in the following polypeptide are cleaved by trypsin? By chymotrypsin? Phe-Leu-Met-Lys-Tyr-Asp-Gly-Gly-Arg-Val-Ile-Pro-Tyr
Problem 26-47
What kinds of reactions do the following classes of enzymes catalyze? (a)
Hydrolases (b)
Lyases (c)
Transferases Problem 26-48
Which of the following amino acids are more likely to be found on the exterior of a globular protein, and which on the interior? Explain.
(a) Valine (b)
Aspartic acid (c) Phenylalanine (d)
Lysine Problem 26-49
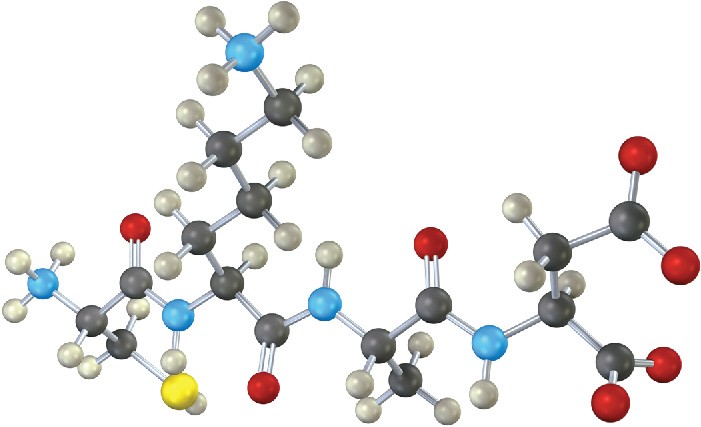
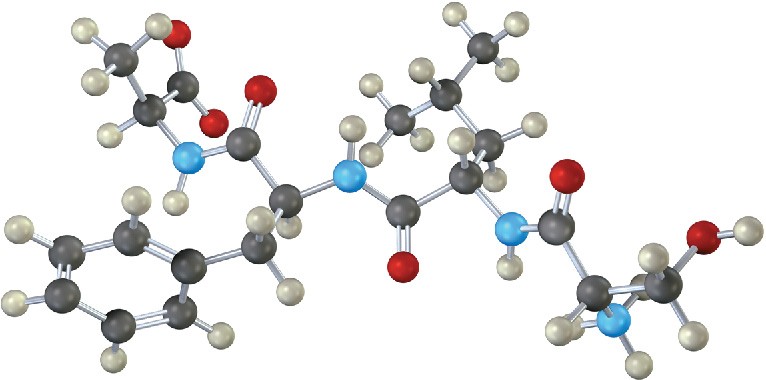
Leuprolide is a synthetic nonapeptide used to treat both endometriosis in women and prostate cancer in men.
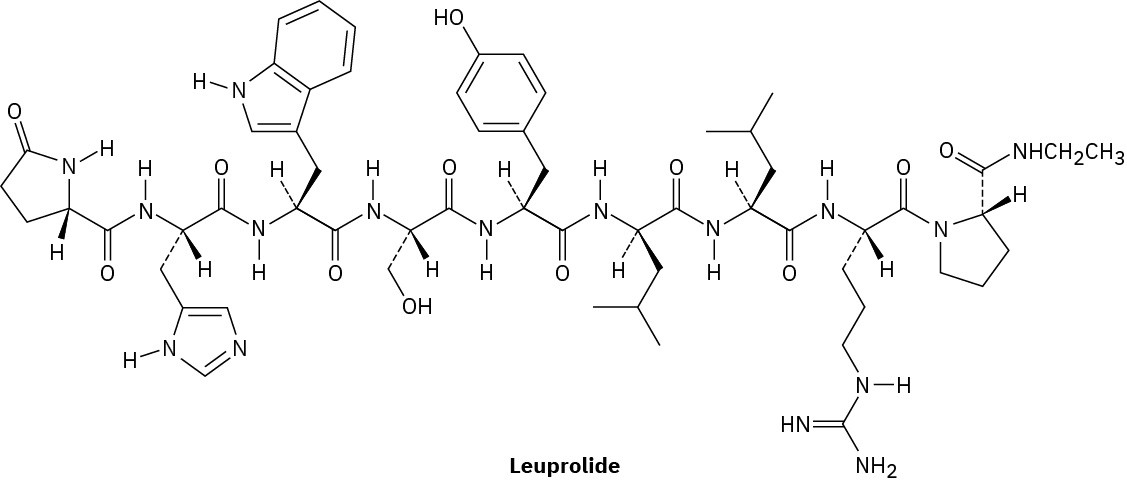
(a)
Both C-terminal and N-terminal amino acids in leuprolide have been structurally modified. Identify the modifications.
(b)
One of the nine amino acids in leuprolide has D stereochemistry rather than the usual L. Which one?
(c)
Write the structure of leuprolide using both one- and three-letter abbreviations. (d)
What charge would you expect leuprolide to have at neutral pH?
General Problems
Problem 26-50
The α-helical parts of myoglobin and other proteins stop whenever a proline residue is encountered in the chain. Why is proline never present in a protein α helix?
Problem 26-51
Arginine, the most basic of the 20 common amino acids, contains a guanidino functional group in its side chain. Explain, using resonance structures to show how the protonated guanidino group is stabilized.
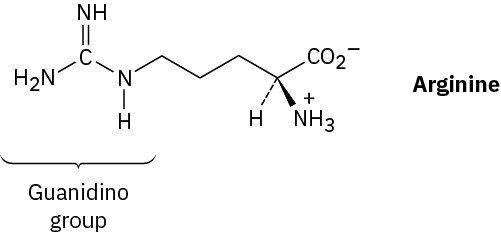
Problem 26-52
Cytochrome c is an enzyme found in the cells of all aerobic organisms. Elemental analysis of cytochrome c shows that it contains 0.43% iron. What is the minimum molecular weight of this enzyme?
Problem 26-53
Evidence for restricted rotation around amide 𝑂═𝐶–𝑁 bonds comes from NMR studies. At room temperature, the 1H NMR spectrum of N,N-dimethylformamide shows three peaks:
2.9 δ (singlet, 3 H), 3.0 δ (singlet, 3 H), and 8.0 δ (singlet, 1 H). As the temperature is raised, however, the two singlets at 2.9 δ and 3.0 δ slowly merge. At 180 °C, the 1H NMR spectrum shows only two peaks: 2.95 δ (singlet, 6 H) and 8.0 δ (singlet, 1 H). Explain this temperature-dependent behavior.

Problem 26-54
Propose a structure for an octapeptide that shows the composition Asp, Gly2, Leu, Phe, Pro2, Val on amino acid analysis. Edman analysis shows a glycine N-terminal group, and leucine is the C-terminal group. Acidic hydrolysis gives the following fragments:
Val-Pro-Leu, Gly, Gly-Asp-Phe-Pro, Phe-Pro-Val Problem 26-55
Look at the structure of human insulin, and indicate where in each chain the molecule is cleaved by trypsin and chymotrypsin.
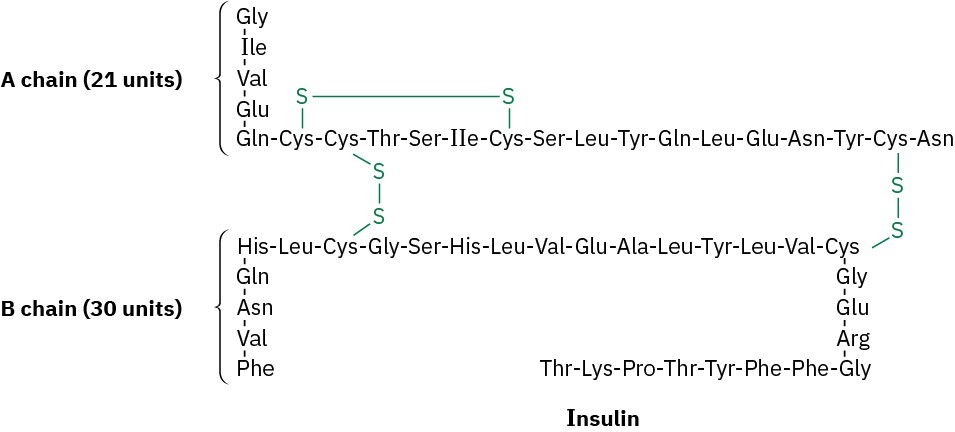
Problem 26-56
What is the structure of a nonapeptide that gives the following fragments when cleaved? Trypsin cleavage: Val-Val-Pro-Tyr-Leu-Arg, Ser-Ile-Arg
Chymotrypsin cleavage: Leu-Arg, Ser-Ile-Arg-Val-Val-Pro-Tyr Problem 26-57
Oxytocin, a nonapeptide hormone secreted by the pituitary gland, functions by stimulating uterine contraction and lactation during childbirth. Its sequence was determined from the following evidence:
- Oxytocin is a cyclic compound containing a disulfide bridge between two cysteine residues.
- When the disulfide bridge is reduced, oxytocin has the constitution Asn, Cys2, Gln, Gly, Ile, Leu, Pro, Tyr.
- Partial hydrolysis of reduced oxytocin yields seven fragments: Asp-Cys, Ile-Glu, Cys-Tyr, Leu-Gly, Tyr-Ile-Glu, Glu-Asp-Cys, and Cys-Pro-Leu.
- Gly is the C-terminal group.
- Both Glu and Asp are present as their side-chain amides (Gln and Asn) rather than as free side-chain acids.
What is the amino acid sequence of reduced oxytocin? What is the structure of oxytocin itself?
Problem 26-58
Aspartame, a nonnutritive sweetener marketed under such trade names as Equal, NutraSweet, and Canderel, is the methyl ester of a simple dipeptide, Asp-Phe-OCH3.
(a)
Draw the structure of aspartame. (b)
The isoelectric point of aspartame is 5.9. Draw the principal structure present in aqueous solution at this pH.
(c)
Draw the principal form of aspartame present at physiological pH = 7.3. Problem 26-59
Refer to Figure 26.5 and propose a mechanism for the final step in Edman degradation— the acid-catalyzed rearrangement of the ATZ derivative to the PTH derivative.
Problem 26-60
Amino acids are metabolized by a transamination reaction in which the −NH2 group of the amino acid changes places with the keto group of an α-keto acid. The products are a new amino acid and a new α-keto acid. Show the product from transamination of isoleucine.
Problem 26-61
The first step in the biological degradation of histidine is formation of a 4-methylidene-5- imidazolone (MIO) by cyclization of a segment of the peptide chain in the histidine ammonia lyase enzyme. Propose a mechanism.
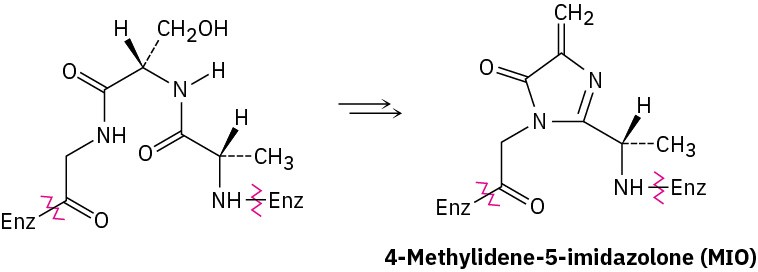
Problem 26-62
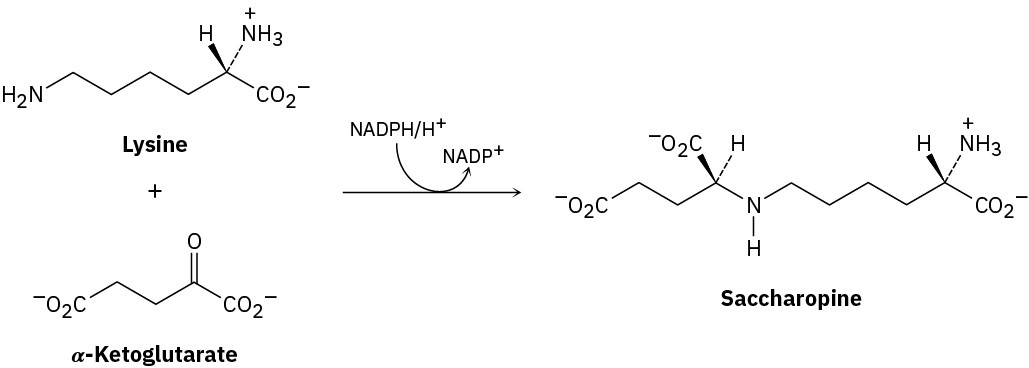
The first step in the biological degradation of lysine is reductive amination with α– ketoglutarate to give saccharopine. Nicotinamide adenine dinucleotide phosphate (NADPH), a relative of NADH, is the reducing agent. Show the mechanism.