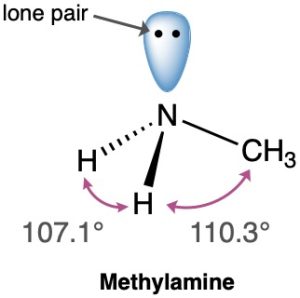
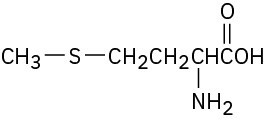The valence-bond concept of orbital hybridization described in the previous four sections is not limited to carbon. Covalent bonds formed by other elements can also be described using hybrid orbitals. Look, for instance, at the nitrogen atom in methylamine (CH3NH2), an organic derivative of ammonia (NH3) and the substance responsible for the odor of rotting fish.
The experimentally measured H–N–H bond angle in methylamine is 107.1°, and the C–N–H bond angle is 110.3°, both of which are close to the 109.5° tetrahedral angle found in methane. We therefore assume that nitrogen forms four sp3-hybridized orbitals, just as carbon does. One of the four sp3 orbitals is occupied by two nonbonding electrons (a lone pair), and the other three hybrid orbitals have one electron each. Overlap of these three half-filled nitrogen orbitals with half-filled orbitals from other atoms (C or H) gives methylamine. Note that the lone pair of electrons in the fourth sp3 hybrid orbital of nitrogen occupies as much space as an N–H bond does and is very important to the chemistry of methylamine and other nitrogen-containing organic molecules.
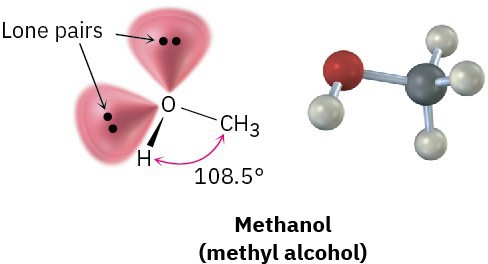
Like the carbon atom in methane and the nitrogen atom in methylamine, the oxygen atom in methanol and many other organic molecules can be described as sp3– hybridized. The C–O–H bond angle in methanol is 108.5°, very close to the 109.5° tetrahedral angle. Two of the four sp3 hybrid orbitals on oxygen are occupied by lone pairs, and two are used to form bonds.
In the periodic table, phosphorus and sulfur are the third-row analogs of nitrogen and oxygen, and the bonding in both can be described using hybrid orbitals. Because of their positions in the third row, however, both phosphorus and sulfur can expand their outer- shell octets and form more than the typical number of covalent bonds. Phosphorus, for instance, often forms three (like N) or five covalent bonds, and sulfur often forms two (like O), four, or six.
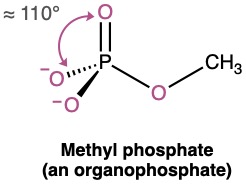
Phosphorus is most commonly encountered in biological molecules in compounds called organophosphates, which contain a phosphorus atom bonded to four oxygens, with one of the oxygens also bonded to carbon. Methyl phosphate, CH3OPO32−, is the simplest example. The O–P–O bond angle in such compounds is typically in the range 110° to 112°, implying sp3 hybridization for phosphorus orbitals.
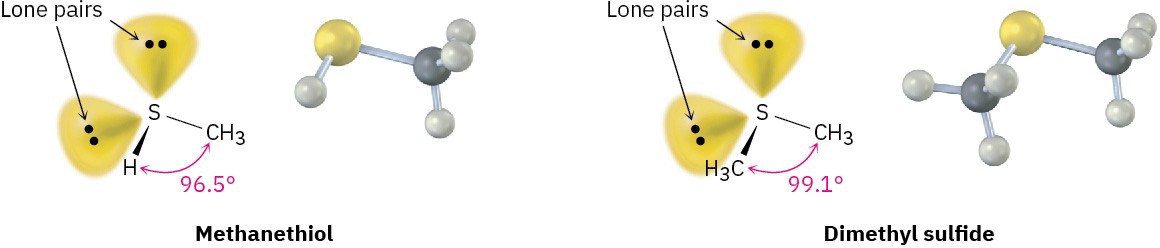
Sulfur is most commonly encountered in biological molecules either in compounds called thiols, which have a sulfur atom bonded to one hydrogen and one carbon, C–S–H or in sulfides, which have a sulfur atom bonded to two carbons, C–S–C. Produced by some bacteria, methanethiol (CH3SH) is the simplest example of a thiol, and dimethyl sulfide, H3C–S–CH3, is the simplest example of a sulfide. Both can be described by approximate sp3 hybridization around sulfur, although both have significant deviation from the 109.5° tetrahedral angle.
Problem 1-14
Identify all nonbonding lone pairs of electrons in the following molecules, and tell what electronic geometry you expect for each of the indicated atoms.
(a) The oxygen atom in dimethyl ether, CH3–O–CH3
(b) The nitrogen atom in trimethylamine, ![]()
(c) The phosphorus atom in phosphine, PH3
(d) The sulfur atom in the amino acid methionine,