Ethylene and propylene, the simplest alkenes, are the two most important organic chemicals produced industrially. Approximately 220 million tons of ethylene and 138 million tons of propylene are produced worldwide each year for use in the synthesis of polyethylene, polypropylene, ethylene glycol, acetic acid, acetaldehyde, and a host of other substances (Figure 7.2).
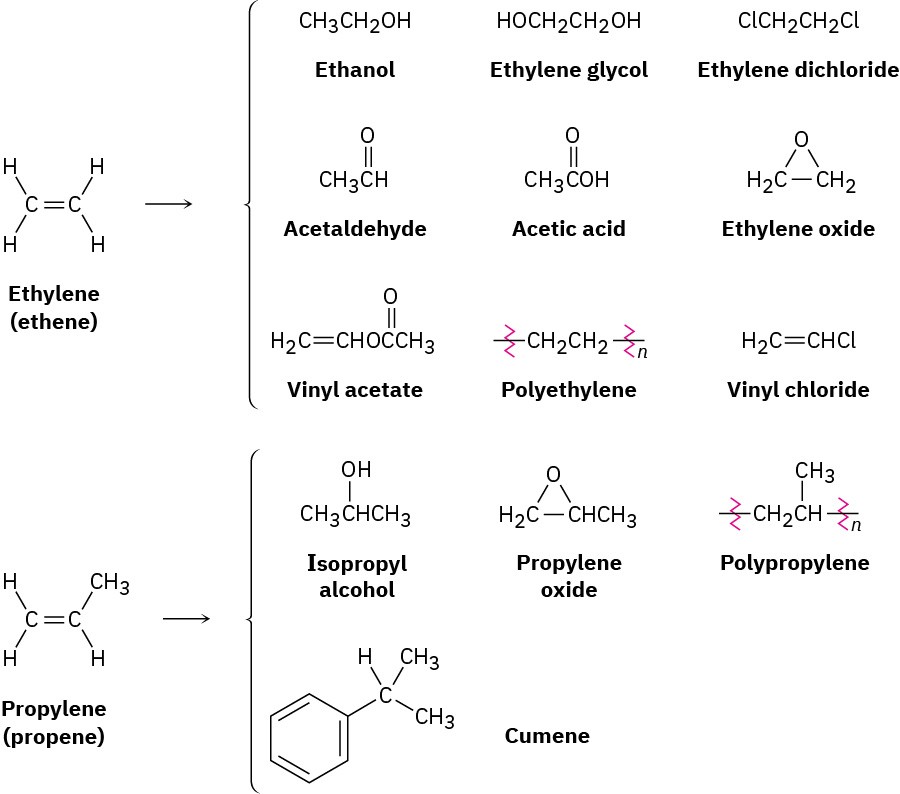
Figure 7.2 Compounds derived industrially from ethylene and propylene. Ethylene, propylene, and butene are synthesized from (C2–C8) alkanes by a process called steam cracking at temperatures up to 900 °C.
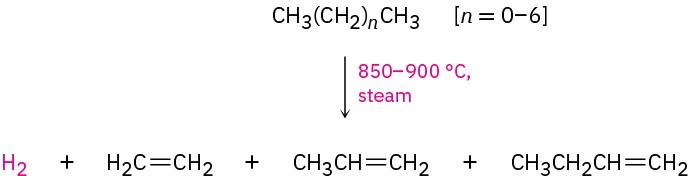
The cracking process is complex, although it undoubtedly involves radical reactions. The high-temperature reaction conditions cause spontaneous breaking of C−C and C−H bonds, with the resultant formation of smaller fragments. We might imagine, for instance, that a molecule of butane splits into two ethyl radicals, each of which then loses a hydrogen atom to generate two molecules of ethylene.
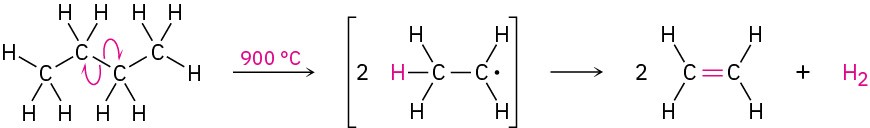
Steam cracking is an example of a reaction whose energetics are dominated by entropy (ΔS°) rather than by enthalpy (ΔH°) in the free-energy equation ΔG° = ΔH° − TΔS° discussed in Section 6.7. Although the bond dissociation energy D for a carbon–carbon single bond is relatively high (about 370 kJ/mol) and cracking is endothermic, the large positive entropy change resulting from the fragmentation of one large molecule into several smaller pieces, together with the high temperature, makes the TΔS° term larger than the ΔH° term, thereby favoring the cracking reaction.

