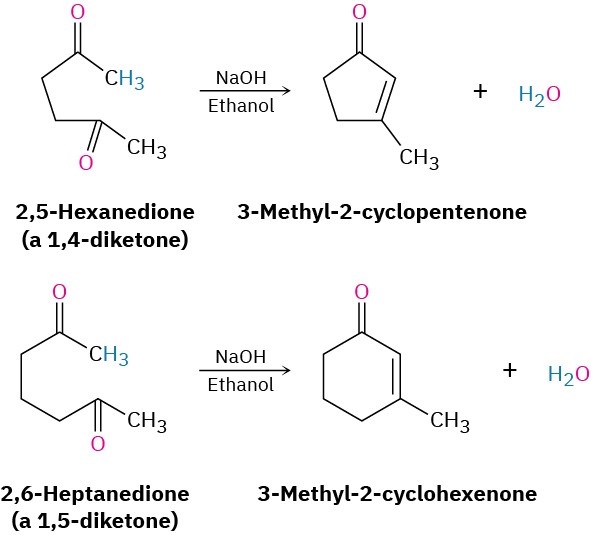
The aldol reactions we’ve seen thus far have all been intermolecular, meaning that they have taken place between two different molecules. When certain dicarbonyl compounds are treated with base, however, an intramolecular aldol reaction can occur, leading to the formation of a cyclic product. For example, base treatment of a 1,4-diketone such as 2,5-hexanedione yields a cyclopentenone product, and base treatment of a 1,5-diketone such as 2,6-heptanedione yields a cyclohexenone.
The mechanism of intramolecular aldol reactions is similar to that of intermolecular reactions. The only difference is that both the nucleophilic carbonyl anion donor and the electrophilic carbonyl acceptor are now in the same molecule. One complication, however, is that intramolecular aldol reactions might lead to a mixture of products, depending on which enolate ion is formed. For example, 2,5-hexanedione might yield either the five-membered-ring product 3-methyl-2-cyclopentenone or the three-membered-ring product (2-methylcyclopropenyl) ethanone (Figure 23.4). In practice, though, only the cyclopentenone is formed.
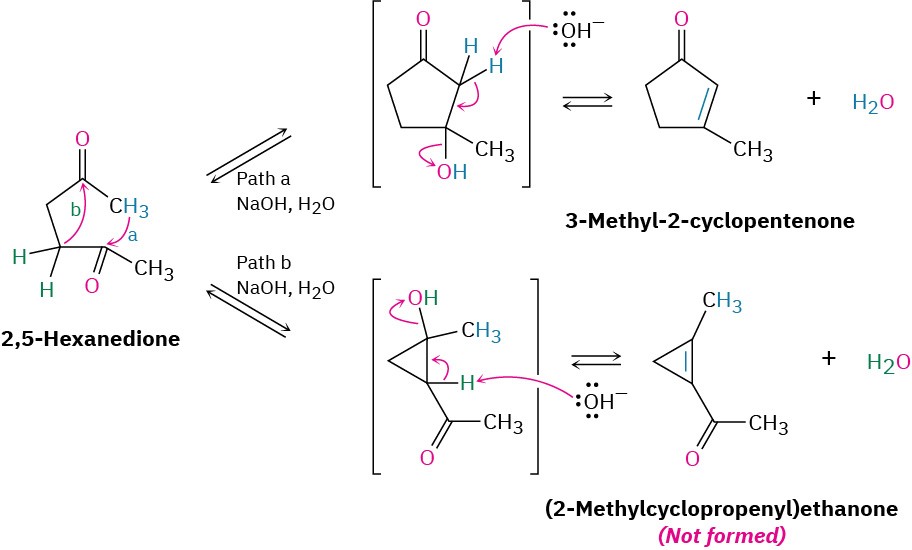 Figure 23.4 Intramolecular aldol reaction of 2,5-hexanedione yields 3-methyl-2-cyclopentenone rather than the alternative cyclopropene.
Figure 23.4 Intramolecular aldol reaction of 2,5-hexanedione yields 3-methyl-2-cyclopentenone rather than the alternative cyclopropene.
The selectivity observed in the intramolecular aldol reaction of 2,5-hexanedione is due to the fact that all steps in the mechanism are reversible, so an equilibrium is reached. Thus, the relatively strain-free cyclopentenone product is considerably more stable than the highly strained cyclopropene alternative. For similar reasons, intramolecular aldol reactions of 1,5-diketones lead only to cyclohexenone products rather than to the more strained acylcyclobutenes.
Problem 23-9
Treatment of a 1,3-diketone such as 2,4-pentanedione with base does not give an aldol condensation product. Explain.
Problem 23-10
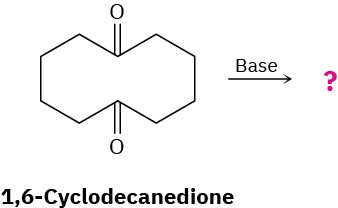
What product would you expect to obtain from base treatment of 1,6-cyclodecanedione?