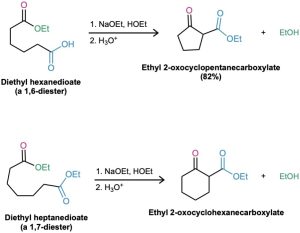
Intramolecular Claisen condensations can be carried out with diesters, just as intramolecular aldol condensations can be carried out with diketones (Section 23.6). Called the Dieckmann cyclization, this reaction works best on 1,6-diesters and 1,7-diesters.
Intramolecular Claisen cyclization of a 1,6-diester gives a five-membered cyclic β-keto ester, and cyclization of a 1,7-diester gives a six-membered cyclic β-keto ester.
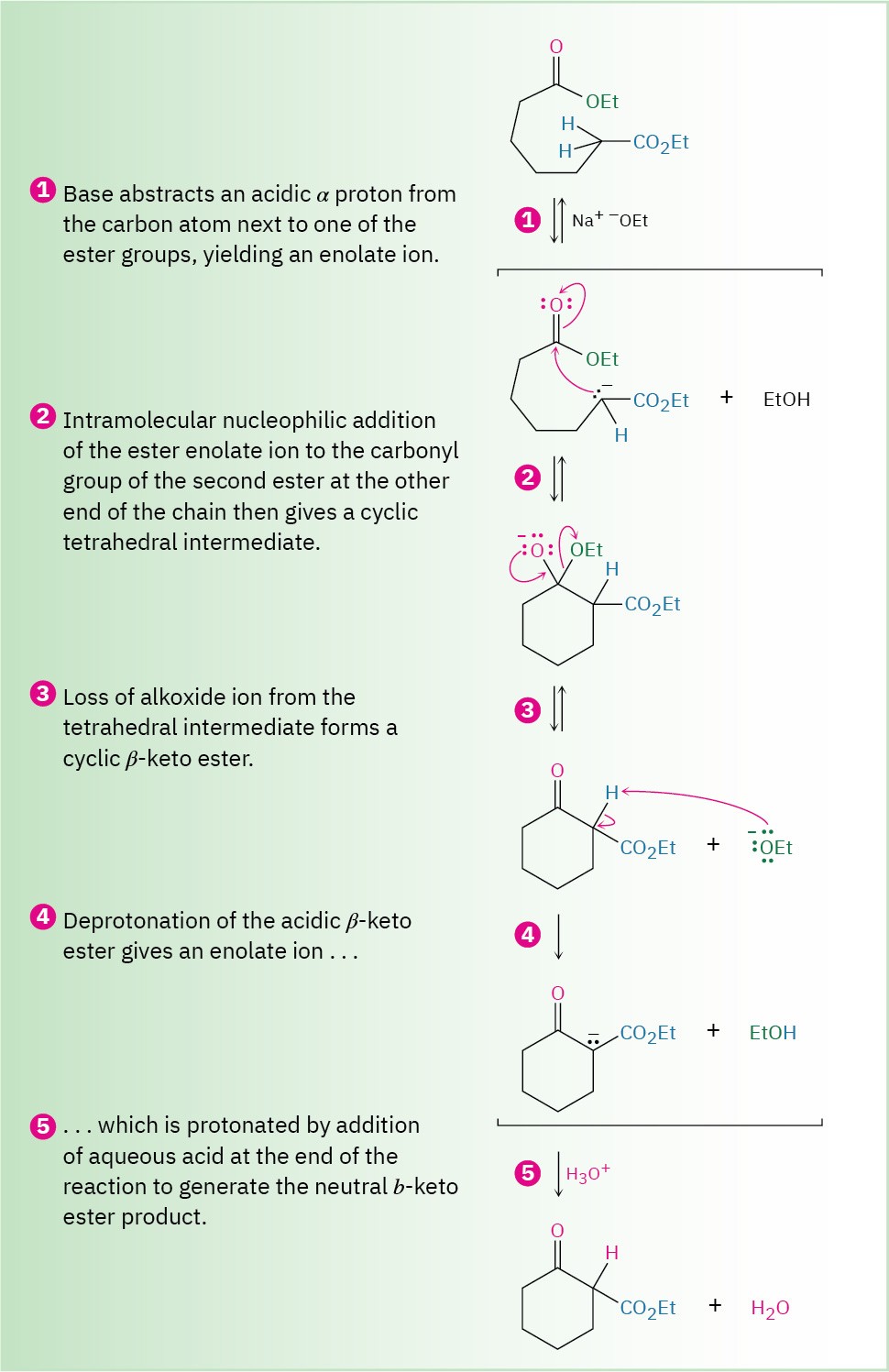
The mechanism of the Dieckmann cyclization, shown in Figure 23.6, is the same as that of the Claisen condensation. One of the two ester groups is converted into an enolate ion, which carries out a nucleophilic acyl substitution on the second ester group at the other end of the molecule. A cyclic β-keto ester product results.
Figure 23.6 Mechanism of the Dieckmann cyclization of a 1,7-diester to yield a cyclic β-keto ester product.
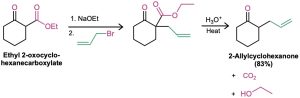
The cyclic β-keto ester produced in a Dieckmann cyclization can be further alkylated and decarboxylated by a series of reactions analogous to those used in the acetoacetic ester synthesis (Section 22.7). Alkylation and subsequent decarboxylation of ethyl 2- oxocyclohexanecarboxylate, for instance, yields a 2-alkylcyclohexanone. The overall sequence of (1) Dieckmann cyclization, (2) β-keto ester alkylation, and (3) decarboxylation is a powerful method for preparing 2-substituted cyclopentanones and cyclohexanones.
Problem 23-14
What product would you expect from the following reaction?

Problem 23-15
Dieckmann cyclization of diethyl 3-methylheptanedioate gives a mixture of two β-keto ester products. What are their structures, and why is a mixture formed?

