A carbonyl compound with a hydrogen atom on its α carbon is in equilibrium with its corresponding enol isomer (Section 9.4). This spontaneous interconversion between two isomers, usually with the change in position of a hydrogen, is called tautomerism, from the Greek tauto, meaning “the same,” and meros, meaning “part.” The individual keto and enol isomers are called tautomers.
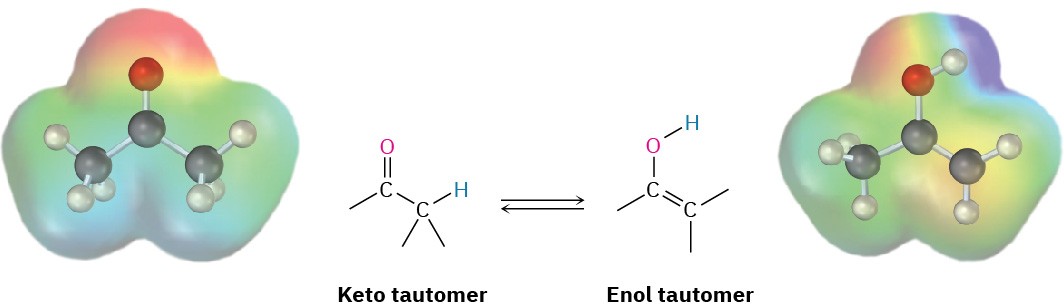
Note the difference between tautomers and resonance forms. Tautomers are constitutional isomers—different compounds with different structures—while resonance forms are different representations of a single compound. Tautomers have their atoms arranged differently, while resonance forms differ only in the position of their π and nonbonding electrons.
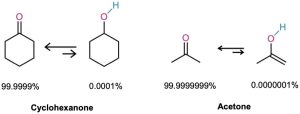
Most monocarbonyl compounds exist almost entirely in their keto form at equilibrium, and it’s usually difficult to isolate the pure enol. Cyclohexanone, for example, contains only about 0.0001% of its enol tautomer at room temperature. The percentage of enol tautomer is even lower for carboxylic acids, esters, and amides. The enol can only predominate when stabilized by conjugation or intramolecular hydrogen bonding. Thus, 2,4-pentanedione is about 76% enol tautomer. Although enols are scarce at equilibrium, they are nevertheless responsible for much of the chemistry of carbonyl compounds because they are so reactive.
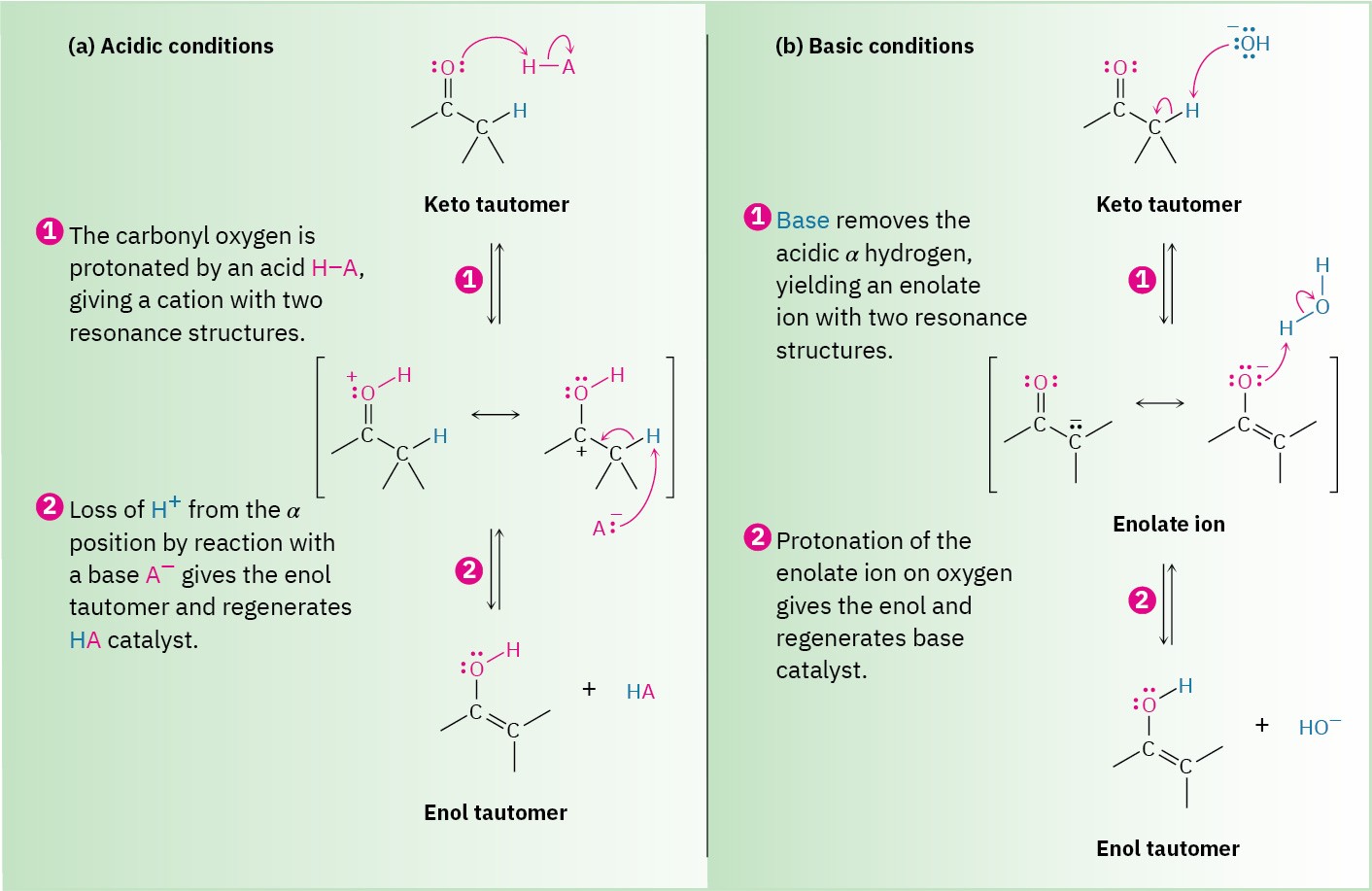
Keto–enol tautomerism of carbonyl compounds is catalyzed by both acids and bases. Acid catalysis occurs by protonation of the carbonyl oxygen atom to give an intermediate cation that loses H+ from its α carbon to yield a neutral enol (Figure 22.2a). This proton loss from the cation intermediate is similar to what occurs during an E1 reaction when a carbocation loses H+ to form an alkene (Section 11.10).
Base-catalyzed enol formation occurs because the presence of a carbonyl group makes the hydrogens on the α carbon weakly acidic. Thus, a carbonyl compound can act as an acid and donate one of its α hydrogens to a sufficiently strong base. The resultant resonance- stabilized anion, an enolate ion, is then protonated to yield a neutral compound. If protonation of the enolate ion takes place on the α carbon, the keto tautomer is regenerated and no net change occurs. If, however, protonation takes place on the oxygen atom, an enol tautomer is formed (Figure 22.2b).
Figure 22.2 Mechanism of enol formation under both acid-catalyzed and base-catalyzed conditions.
(a) Acid catalysis involves (1) initial protonation of the carbonyl oxygen followed by (2) removal of H+ from the α position.
(b) Base catalysis involves (1) initial deprotonation of the α position to give an enolate ion, followed by (2) reprotonation on oxygen.
Note that only the hydrogens on the α position of carbonyl compounds are acidic. Hydrogens at β, γ, δ, and so on, aren’t acidic and can’t be removed by base because the resulting anions can’t be resonance-stabilized by the carbonyl group.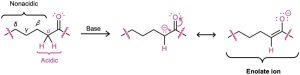
Problem 22-1
Draw structures for the enol tautomers of the following compounds:
(a) cyclopentanone
(b) methyl thioacetate
(c) ethyl acetate
(d) propanal
(e) acetic acid
(f) phenylacetone
Problem 22-2
How many acidic hydrogens does each of the molecules listed in Problem 22-1 have? Identify them.
Problem 22-3
Draw structures for all monoenol forms of the following molecule. Which would you expect to be most stable? Explain.