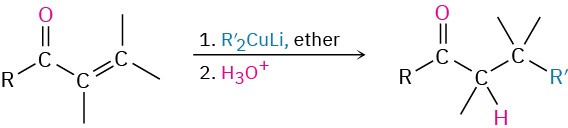- acetal, R2C(OR′)2
- acyl group
- 1,2-addition
- 1,4-addition
- aldehyde (RCHO)
- Cannizzaro reaction
- carbinolamine
- conjugate addition
- cyanohydrin
- enamine (R2N–CR═CR2)
- hemiacetal
- imine (R2C═NR)
- ketal
- ketone (R2CO)
- McLafferty rearrangement
- nucleophilic addition reaction
- phosphorane
- Schiff bases
- Wittig reaction
- Wolff–Kishner reaction
- ylide
- Preparation of aldehydes (Section 19.2)
- Oxidation of primary alcohols (Section 17.7)
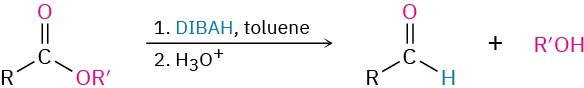
- Partial reduction of esters (Section 19.2)
- Preparation of ketones
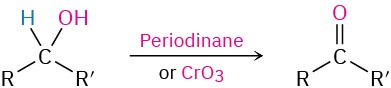
- Oxidation of secondary alcohols (Section 17.7)
- Diorganocopper reaction with acid chlorides (Section 19.2)
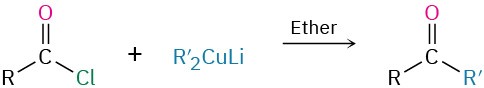
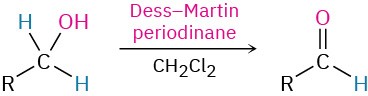
- Oxidation of aldehydes (Section 19.3)
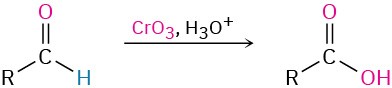
- Nucleophilic addition reactions of aldehydes and ketones
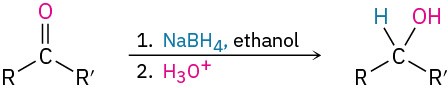
- Addition of hydride to give alcohols: reduction (Section 19.7)
- Addition of Grignard reagents to give alcohols (Section 19.7)
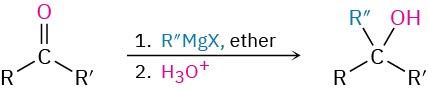
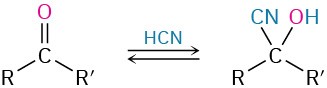
- Addition of HCN to give cyanohydrins (Section 19.6)
- Addition of primary amines to give imines (Section 19.8)
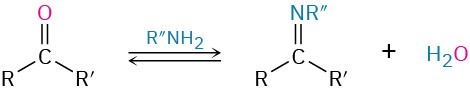
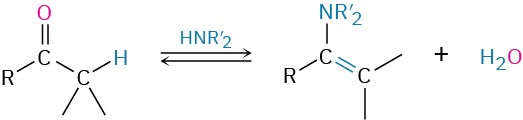
- Addition of secondary amines to give enamines (Section 19.8)
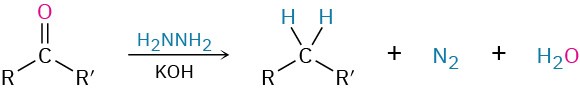
- Wolff–Kishner reaction to give alkanes (Section 19.9)
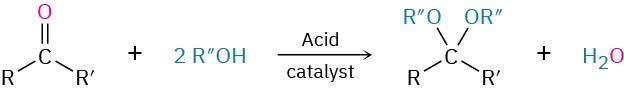
- Addition of alcohols to give acetals (Section 19.10)
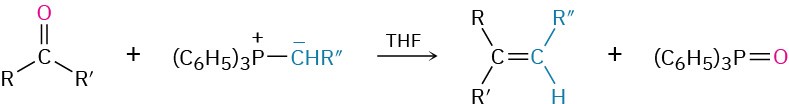
- Addition of phosphorus ylides to give alkenes: Wittig reaction (Section 19.11)
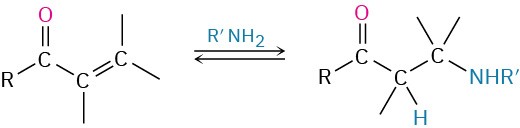
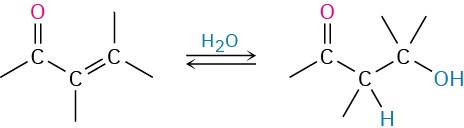
- Conjugate additions to α,β-unsaturated aldehydes and ketones (Section 19.13)
- Conjugate addition of amines
- Conjugate addition of water
- Conjugate addition of alkyl groups by diorganocopper reaction