- alkylamine
- amine
- arylamine
- azo compound (R–N=N–R′)
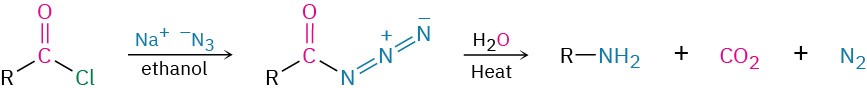
- Curtius rearrangement
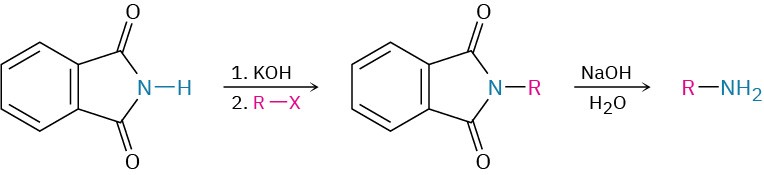
- Gabriel amine synthesis
- heterocyclic amine
- Hofmann elimination reaction
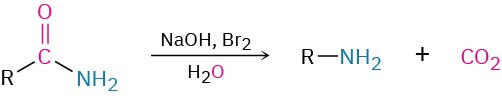
- Hofmann rearrangement
- imide (O=C–N–C=O)
- primary amine (RNH2)
- quaternary ammonium salt
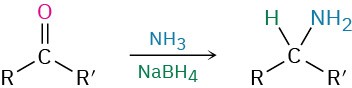
- reductive amination
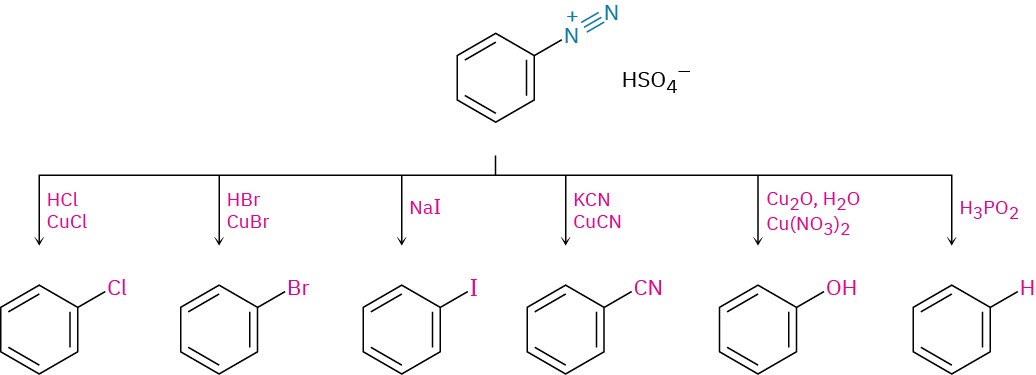
- Sandmeyer reaction
- secondary amine (R2NH)
- tertiary amine (R3N)
Summary of Reactions
- Synthesis of amines (Section 24.6)
- Reduction of nitriles
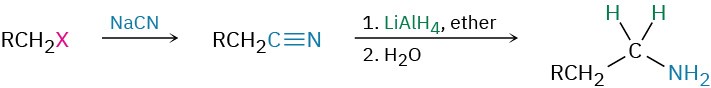
- Reduction of amides
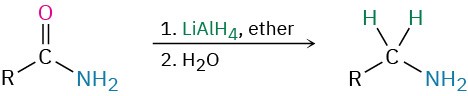
- Reduction of nitrobenzenes
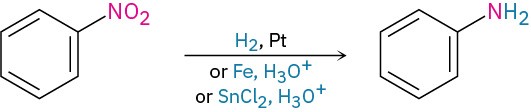
- SN2 Alkylation of alkyl halides
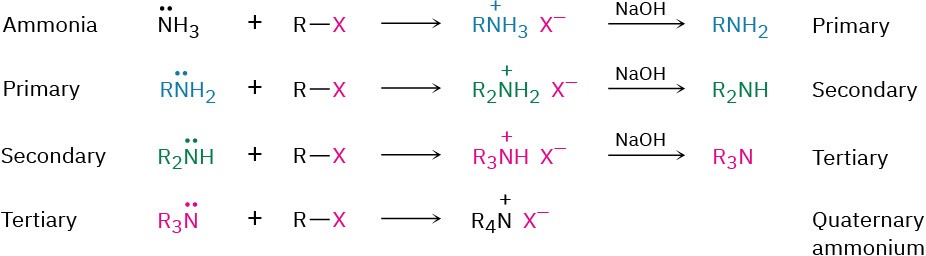
- Gabriel amine synthesis
- Reduction of azides
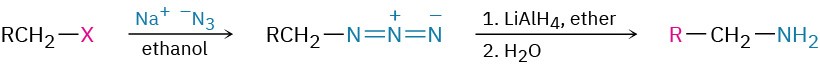
- Reductive amination of aldehydes/ketones
- Hofmann rearrangement of amides
- Curtius rearrangement of acyl azides
- Reactions of amines
- Alkylation with alkyl halides; see reaction 1(d)
- Hofmann elimination (Section 24.7)
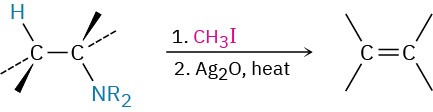
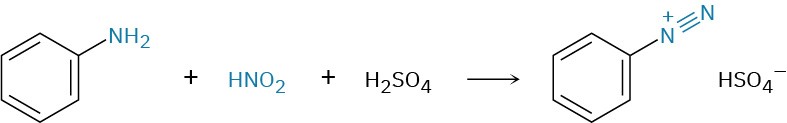
- Diazotization (Section 24.8)
- Reactions of arenediazonium salts (Section 24.8)
- Nucleophilic substitutions
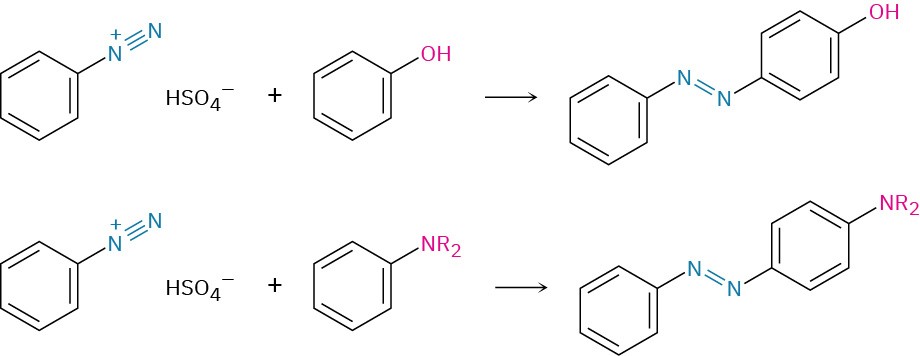
- Diazonium coupling