Electrophilic addition to a conjugated diene at or below room temperature normally leads to a mixture of products in which the 1,2 adduct predominates over the 1,4 adduct. When the same reaction is carried out at higher temperatures, however, the product ratio often changes and the 1,4 adduct predominates. For example, addition of HBr to 1,3-butadiene at 0 °C yields a 71 : 29 mixture of 1,2 and 1,4 adducts, but the same reaction carried out at 40°C yields a 15 : 85 mixture. Furthermore, when the product mixture formed at 0 °C is heated to 40 °C in the presence of HBr, the ratio of adducts slowly changes from 71 : 29 to 15 : 85. Why?

To understand the effect of temperature on product distribution, let’s briefly review what we said in Section 6.7 about rates and equilibria. Imagine a reaction that can give either or both of two products, B and C.
Let’s assume that B forms faster than C (in other words, Δ𝐺‡B < Δ𝐺‡C) but that C is more stable than B (in other words, Δ𝐺∘C > Δ𝐺∘B). An energy diagram for the two processes might look like that shown in Figure 14.6.
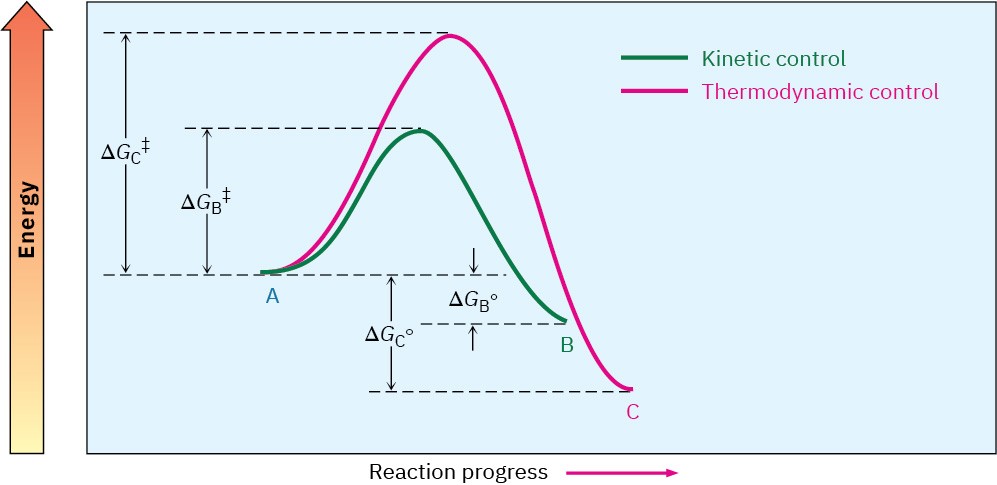
Figure 14.6 An energy diagram for two competing reactions in which the less stable product B forms faster than the more stable product C.
Let’s first carry out the reaction at a lower temperature so that both processes are irreversible and no equilibrium is reached. Because B forms faster than C, B is the major product. It doesn’t matter that C is more stable than B, because the two are not in equilibrium. The product of an irreversible reaction depends only on relative rates, not on stability. Such reactions are said to be under kinetic control.

Now let’s carry out the same reaction at some higher temperature so that both processes are readily reversible and an equilibrium is reached. Because C is more stable than B, C is the major product obtained. It doesn’t matter that C forms more slowly than B, because the two are in equilibrium. The product of a readily reversible reaction depends only on stability, not on relative rates. Such reactions are said to be under equilibrium control, or thermodynamic control.

We can now explain the effect of temperature on the electrophilic addition reactions of conjugated dienes. At low temperature (0 °C), HBr adds to 1,3-butadiene under kinetic control to give a 71 : 29 mixture of products, with the more rapidly formed 1,2 adduct predominating. Because these low-temperature conditions don’t allow the reaction to reach equilibrium, the product that forms faster predominates. At higher temperature (40°C), however, the reaction occurs under thermodynamic control to give a 15 : 85 mixture of products, with the more stable 1,4 adduct predominating. The higher temperature allows the addition process to become reversible, so an equilibrium mixture of products results.
Figure 14.7 shows this situation in an energy diagram.
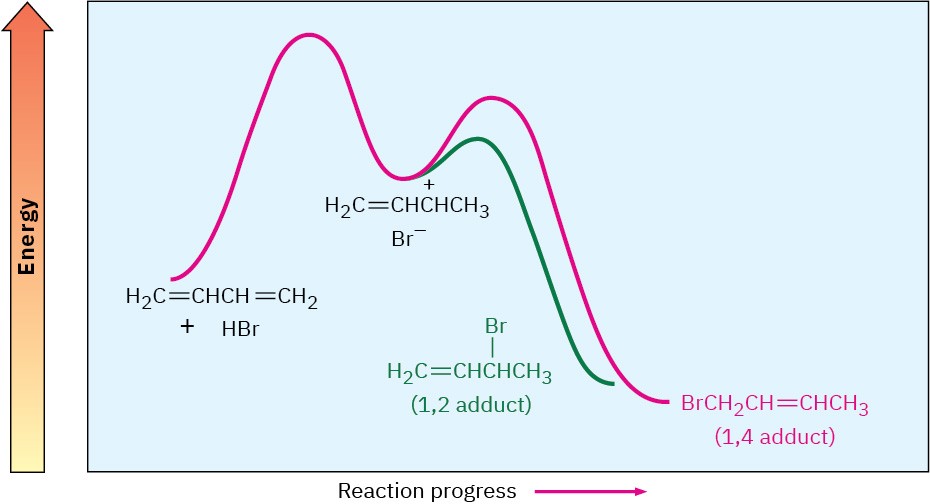
Figure 14.7 Energy diagram for the electrophilic addition of HBr to 1,3-butadiene. The 1,2 adduct is the kinetic product because it forms faster, but the 1,4 adduct is the thermodynamic product because it is more stable.
The electrophilic addition of HBr to 1,3-butadiene is a good example of how a change in experimental conditions can change the product of a reaction. The concept of thermodynamic control versus kinetic control is a useful one that we can sometimes take advantage of in the laboratory.
Problem 14-5
The 1,2 adduct and the 1,4 adduct formed by reaction of HBr with 1,3-butadiene are in equilibrium at 40 °C. Propose a mechanism by which the interconversion of products takes place.
Problem 14-6
Why do you suppose 1,4 adducts of 1,3-butadiene are generally more stable than 1,2 adducts?

