In earlier times, when relatively few pure organic chemicals were known, new compounds were named at the whim of their discoverer. Thus, urea (CH4N2O) is a crystalline substance isolated from urine; morphine (C17H19NO3) is an analgesic (painkiller) named after Morpheus, the Greek god of dreams; and acetic acid, the primary organic constituent of vinegar, is named from the Latin word for vinegar, acetum.
As the science of organic chemistry slowly grew in the 19th century, so too did the number of known compounds and the need for a systematic method of naming them. The system of naming (nomenclature) we’ll use in this book is that devised by the International Union of Pure and Applied Chemistry (IUPAC, usually spoken as eye-you-pac).
A chemical name typically has four parts in the IUPAC system: parent, prefix, locant, and suffix. The parent name identifies the main part of the molecule and tells how many carbon atoms are in that part. Prefixes identify the various substituent groups attached to the parent. Locants give the positions of the attached substituents. And the suffix identifies the primary functional group attached to the parent.

As we cover new functional groups in later chapters, the applicable IUPAC rules of nomenclature will be given. In addition, Appendix A at the back of this book gives an overall view of organic nomenclature and shows how compounds that contain more than one functional group are named. (If preferred, you can study that appendix now.) For the present, let’s see how to name branched-chain alkanes and learn some general rules that are applicable to all compounds.
All but the most complex branched-chain alkanes can be named by following four steps. For a very few compounds, a fifth step is needed.
STEP 1
Identify the parent hydrocarbon.
- Find the longest continuous chain of carbon atoms in the molecule, and use the name of that chain as the parent name. The longest chain may not always be apparent from the manner of writing; you may have to “turn corners.”
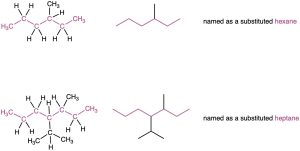
- If two different chains of equal length are present, choose the one with the larger number of branch points as the parent.
STEP 2
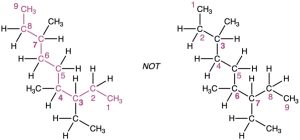
Number the atoms in the longest chain.
- Beginning at the end nearer the first branch point, number each carbon atom in the parent chain.

The first branch occurs at C3 in the proper system of numbering, not at C4.
- If there is branching an equal distance away from both ends of the parent chain, begin numbering at the end nearer the second branch point.
STEP 3
Identify and number the substituents.
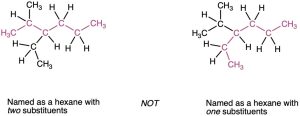
- Assign a number to each substituent to locate its point of attachment to the parent chain.
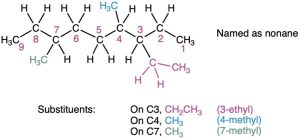
- If there are two substituents on the same carbon, give both the same number. There must be as many numbers in the name as there are substituents.
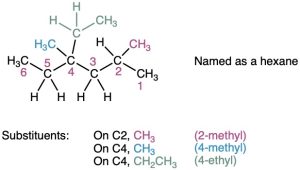
STEP 4
Write the name as a single word.
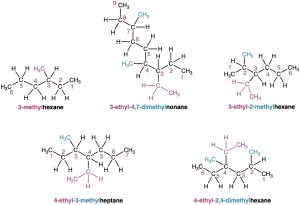
Use hyphens to separate the different prefixes, and use commas to separate numbers. If two or more different substituents are present, cite them in alphabetical order. If two or more identical substituents are present on the parent chain, use one of the multiplier prefixes di-, tri-, tetra-, and so forth, but don’t use these prefixes for alphabetizing. Full names for some of the examples we have been using are as follows:
STEP 5
Name a branched substituent as though it were itself a compound.
In some particularly complex cases, a fifth step is necessary. It occasionally happens that a substituent on the main chain is itself branched. In the following case, for instance, the substituent at C6 is a three-carbon chain with a methyl group. To name the compound fully, the branched substituent must first be named.
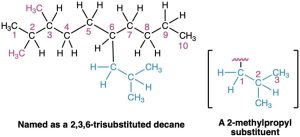
Number the branched substituent beginning at the point of its attachment to the main chain, and identify it—in this case, a 2-methylpropyl group. The substituent is treated as a whole and is alphabetized according to the first letter of its complete name, including any numerical prefix. It is set off in parentheses when naming the entire molecule.
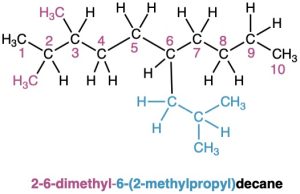
As a further example:
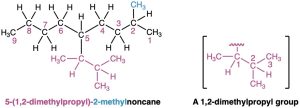
For historical reasons, some of the simpler branched-chain alkyl groups also have nonsystematic, common names, as noted earlier.
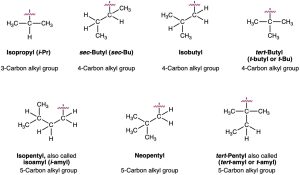
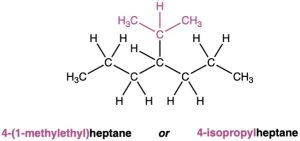
The common names of these simple alkyl groups are so well entrenched in the chemical literature that IUPAC rules make allowance for them. Thus, the following compound is properly named either 4-(1-methylethyl)heptane or 4-isopropylheptane. There’s no choice but to memorize these common names; fortunately, there are only a few of them.
When writing an alkane name, the nonhyphenated prefix iso- is considered part of the alkyl-group name for alphabetizing purposes, but the hyphenated and italicized prefixes sec– and tert– are not. Thus, isopropyl and isobutyl are listed alphabetically under i, but sec– butyl and tert-butyl are listed under b.
Worked Example 3.2 Naming Alkanes
What is the IUPAC name for the following alkane?
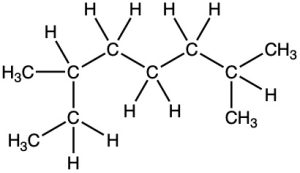
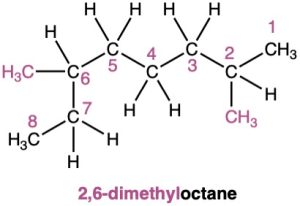
Strategy. Find the longest continuous carbon chain in the molecule, and use that as the parent name. This molecule has a chain of eight carbons—octane—with two methyl substituents. (You have to turn corners to see it.) Numbering from the end nearer the first methyl substituent indicates that the methyls are at C2 and C6.
Solution
Worked Example 3.3 Converting a Chemical Name into a Structure
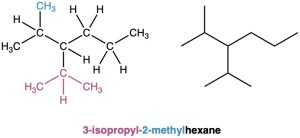
Draw the structure of 3-isopropyl-2-methylhexane.
Strategy. This is the reverse of Worked Example 3.2 and uses a reverse strategy. Look at the parent name (hexane), and draw its carbon structure.
C–C–C–C–C–C Hexane
Next, find the substituents (3-isopropyl and 2-methyl), and place them on the proper carbons.

Finally, add hydrogens to complete the structure.
Problem 3-11
Give IUPAC names for the three isomers of C5H12
Problem 3-12
Draw structures corresponding to the following IUPAC names:
(a) 3,4-Dimethylnonane
(b) Ethyl-4,4-dimethylheptane
(c) 2,2-Dimethyl-4-propyloctane
(d) 2,2,4-Trimethylpentane
Problem 3-13
Name the eight 5-carbon alkyl groups you drew in Problem 3-7.
Problem 3-14
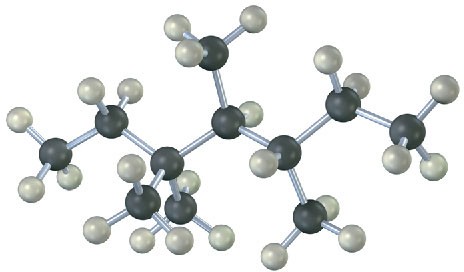
Give the IUPAC name for the following hydrocarbon, and convert the drawing into a skeletal structure.