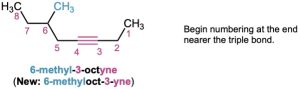
Alkyne nomenclature follows the general rules for hydrocarbons discussed in Section 3.4 and Section 7.3. The suffix -yne is used, and the position of the triple bond is indicated by giving the number of the first alkyne carbon in the chain. Numbering the main chain begins at the end nearer the triple bond so that the triple bond receives as low a number as possible.
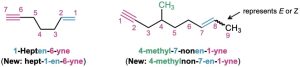
Compounds with more than one triple bond are called diynes, triynes, and so forth; compounds containing both double and triple bonds are called enynes (not ynenes). Numbering of an enyne chain starts from the end nearer the first multiple bond, whether double or triple. When there is a choice in numbering, double bonds receive lower numbers than triple bonds. For example:
As with alkyl and alkenyl substituents derived from alkanes and alkenes, respectively, alkynyl groups are also possible.
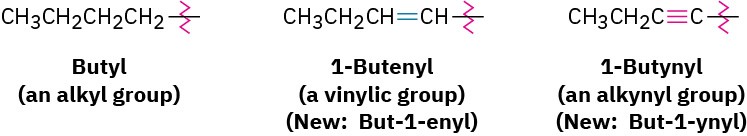
Problem 9-1
Name the following alkynes:
(a)
![]()
(b)
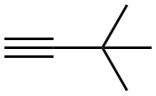
(c)
![]()
(d)
![]()
(e)
![]()
Problem 9-2
There are seven isomeric alkynes with the formula C6H10. Draw and name them.

