Amines can be either alkyl-substituted (alkylamines) or aryl-substituted (arylamines). Although much of the chemistry of the two classes is similar, there are also substantial differences. Amines are classified as primary (RNH2), secondary (R2NH), or tertiary (R3N), depending on the number of organic substituents attached to nitrogen. Thus, methylamine (CH3NH2) is a primary amine, dimethylamine [(CH3)2NH] is a secondary amine, and trimethylamine [(CH3)3N] is a tertiary amine. Note that this usage of the terms primary, secondary, and tertiary differs from our previous usage. When we speak of a tertiary alcohol or alkyl halide, we refer to the degree of substitution at the alkyl carbon atom, but when we speak of a tertiary amine, we refer to the degree of substitution at the nitrogen atom.
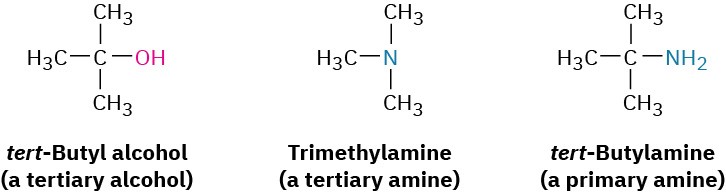
Compounds containing a nitrogen atom with four attached groups also exist, but the nitrogen atom must carry a formal positive charge. Such compounds are called quaternary ammonium salts.

Primary amines are named in the IUPAC system in several ways. For simple amines, the suffix –amine is added to the name of the alkyl substituent. You might also recall from the chapter on Benzene and Aromaticity that the aromatic phenylamine, H2N–C6H5, has the common name aniline.
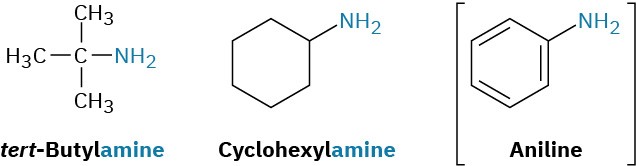
Alternatively, the suffix –amine can be used in place of the final –e in the name of the parent compound.

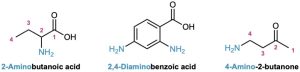
Amines with more than one functional group are named by considering the −NH2 as an amino substituent on the parent molecule.
Symmetrical secondary and tertiary amines are named by adding the prefix di– or tri– to the alkyl group.
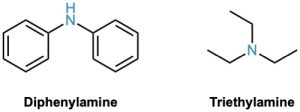
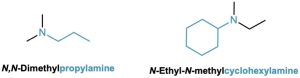
Unsymmetrically substituted secondary and tertiary amines are referred to as N-substituted primary amines. The largest alkyl group takes the parent name, and the other alkyl groups are considered N-substituents on the parent (N because they’re attached to nitrogen).
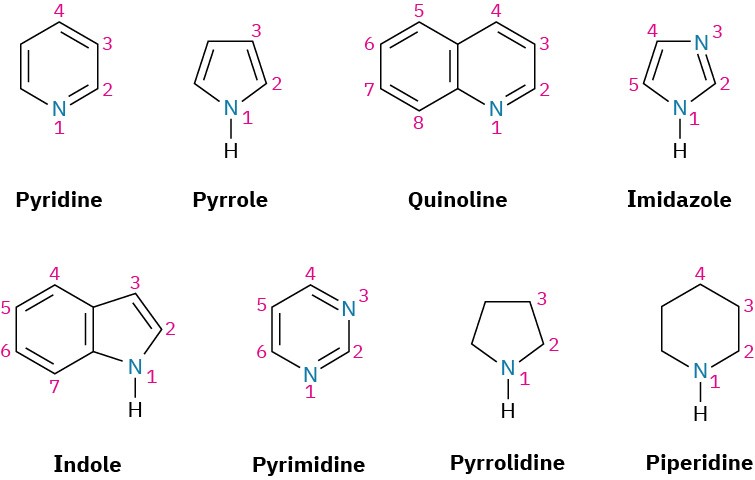
Heterocyclic amines—compounds in which the nitrogen atom occurs as part of a ring—are also common, and each different heterocyclic ring system has its own parent name. The heterocyclic nitrogen atom is always numbered as position 1.
Problem 24-1
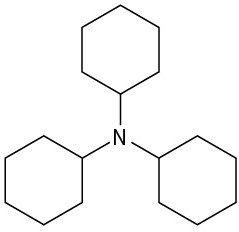
Name the following compounds:
(a)
![]()
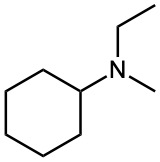
(b)
(c)
(d)
![]()
(e)
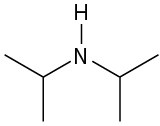
(f)
![]()
Problem 24-2
Draw structures corresponding to the following IUPAC names:
(a) Triisopropylamine
(b) Triallylamine
(c) N-Methylaniline
(d) N-Ethyl-N-methylcyclopentylamine
(e) N-Isopropylcyclohexylamine
(f) N-Ethylpyrrole
Problem 24-3
Draw structures for the following heterocyclic amines:
(a) 5-Methoxyindole
(b) 1,3-Dimethylpyrrole
(c) 4-(N,N-Dimethylamino)pyridine
(d) 5-aminopyrimidine

