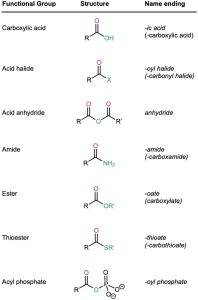
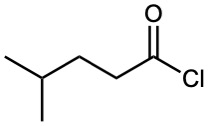
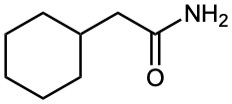
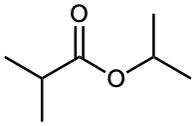
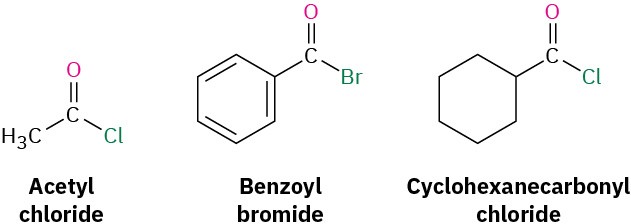
Acid Halides
Acid halides are named by identifying first the acyl group and then the halide. As described in Section 20.1 and shown in Table 20.1, the acyl group name is derived from the carboxylic acid name by replacing the –ic acid or –oic acid ending with –oyl, or the –carboxylic acid ending with –carbonyl. To keep things interesting, however, IUPAC recognizes eight exceptions for which a –yl rather than an –oyl ending is used: formic (formyl), acetic (acetyl), propionic (propionyl), butyric (butyryl), oxalic (oxalyl), malonic (malonyl), succinic (succinyl), and glutaric (glutaryl).
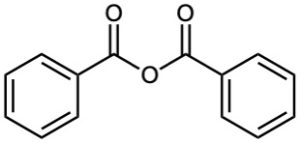
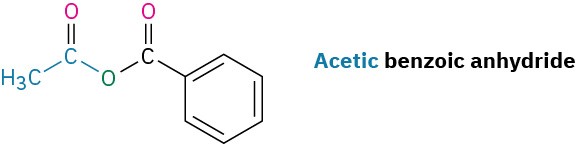
Acid Anhydrides
Symmetrical anhydrides of unsubstituted monocarboxylic acids and cyclic anhydrides of dicarboxylic acids are named by replacing the word acid with anhydride.
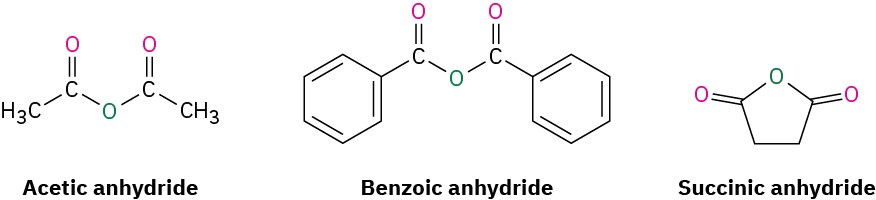
Unsymmetrical anhydrides—those prepared from two different carboxylic acids—are named by listing the two acids alphabetically and then adding anhydride.
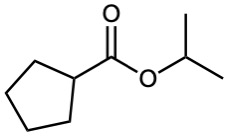
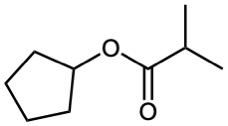
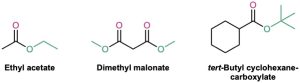
Esters
Esters are named by first identifying the alkyl group attached to oxygen and then the carboxylic acid, with the –ic acid ending replaced by –ate.
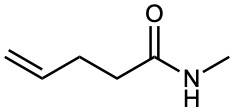
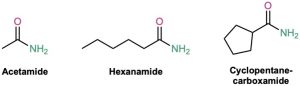
Amides
Amides with an unsubstituted –NH2 group are named by replacing the –oic acid or –ic acid ending with –amide, or by replacing the –carboxylic acid ending with –carboxamide.
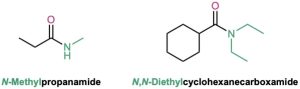
If the nitrogen atom is further substituted, the compound is named by first identifying the substituent groups and then the parent amide. The substituents are preceded by the letter N to identify them as being directly attached to nitrogen.
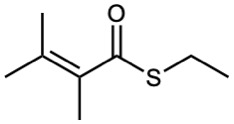
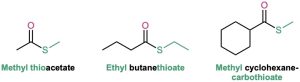
Thioesters
Thioesters are named like the corresponding esters. If the related ester has a common name, the prefix thio– is added to the name of the carboxylate: acetate becomes thioacetate, for instance. If the related ester has a systematic name, the –oate or –carboxylate ending is replaced by –thioate or –carbothioate: butanoate becomes butanethioate and cyclohexanecarboxylate becomes cyclohexanecarbothioate, for instance.
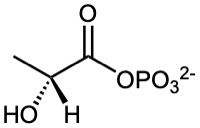
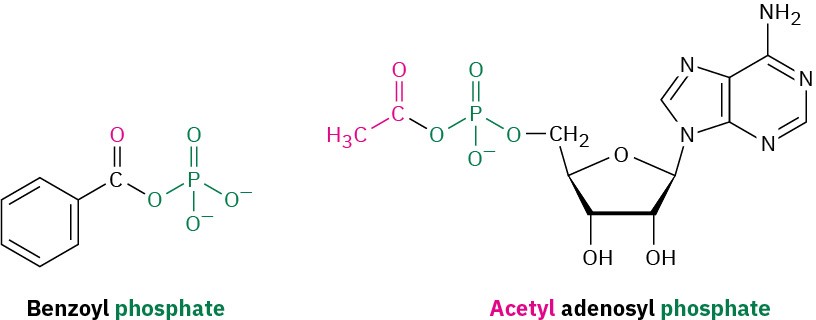
Acyl Phosphates
Acyl phosphates are named by citing the acyl group and adding the word phosphate. If an alkyl group is attached to one of the phosphate oxygens, it is identified after the name of the acyl group. In biological chemistry, acyl adenosyl phosphates are particularly common.
A summary of nomenclature rules for carboxylic acid derivatives is given in Table 21.1. Table 21.1 Nomenclature of Carboxylic Acid Derivatives