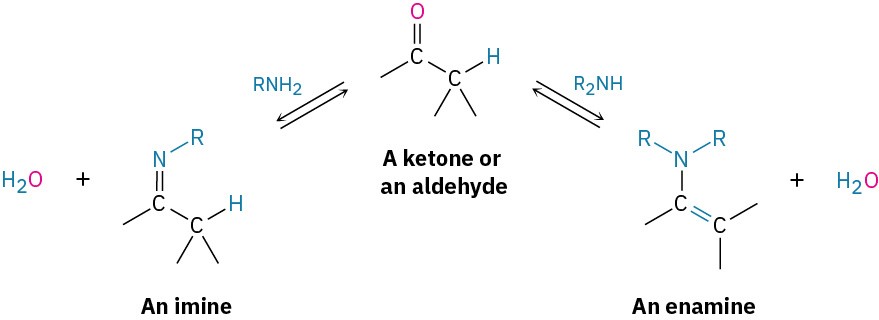
Primary amines, RNH2, add to aldehydes and ketones to yield imines, R2C═NR. Secondary amines, R2NH, add similarly to yield enamines, R2N–CR═CR2 (ene + amine = unsaturated amine).
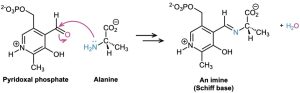
Imines are particularly common as intermediates in biological pathways, where they are often called Schiff bases. The amino acid alanine, for instance, is metabolized in the body by reaction with the aldehyde pyridoxal phosphate (PLP), a derivative of vitamin B6, to yield a Schiff base that is further degraded.
Imine formation and enamine formation seem different because one leads to a product with a C═N bond and the other leads to a product with a C═C bond. Actually, though, the reactions are quite similar. Both are typical examples of nucleophilic addition reactions in which water is eliminated from the initially formed tetrahedral intermediate and a new C═Nu double bond is formed.
Imines are formed in a reversible, acid-catalyzed process (Figure 19.7) that begins with nucleophilic addition of the primary amine to the carbonyl group, followed by transfer of a proton from nitrogen to oxygen to yield a neutral amino alcohol, or carbinolamine.
Protonation of the carbinolamine oxygen by an acid catalyst then converts the –OH into a better leaving group (–OH2+), and E1-like loss of water produces an iminium ion. Loss of a proton from nitrogen gives the final product and regenerates the acid catalyst.
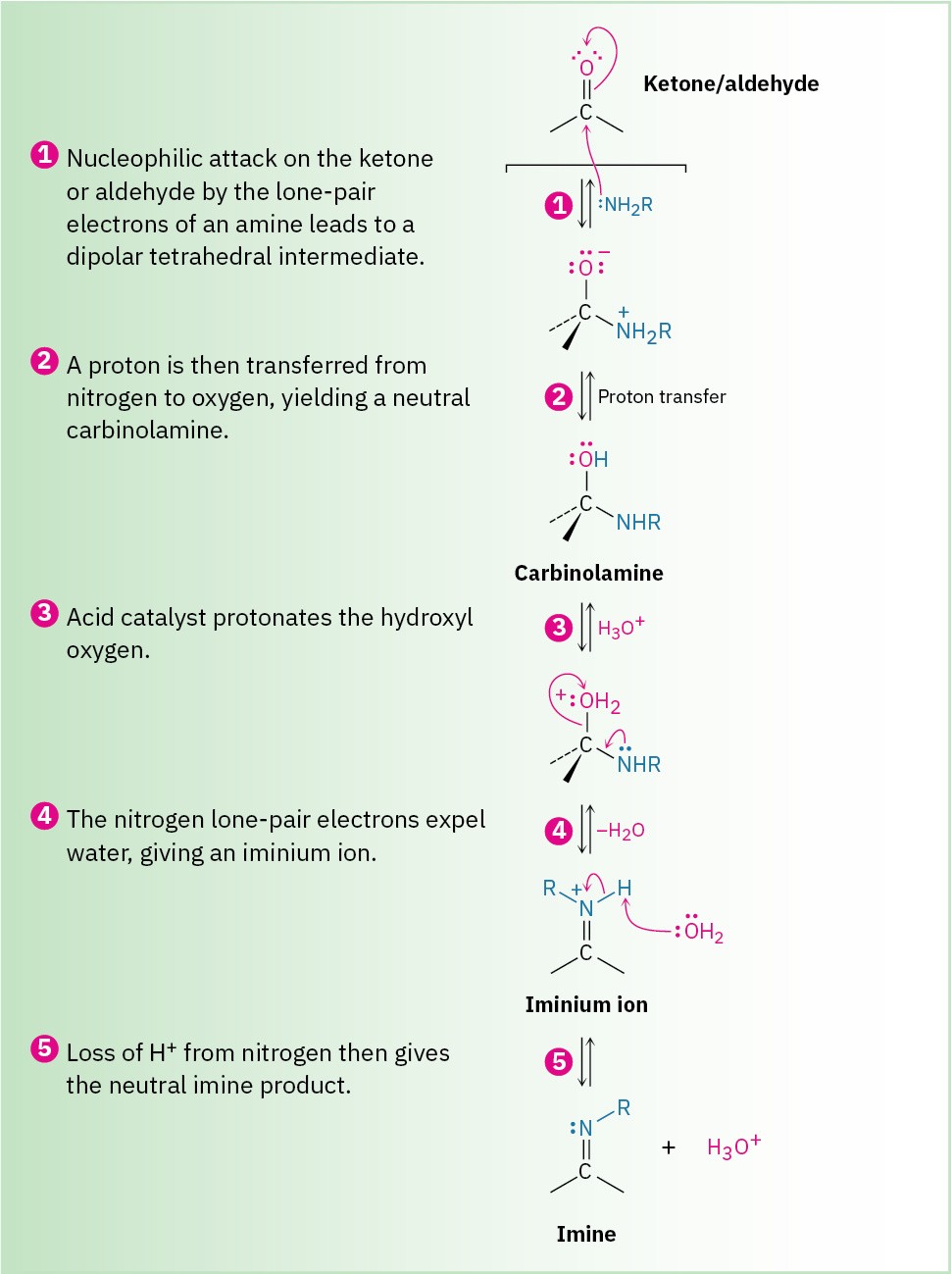
Figure 19.7 Mechanism of imine formation by reaction of an aldehyde or ketone with a primary amine. The key step is the initial nucleophilic addition to yield a carbinolamine intermediate, which then loses water to give the imine.
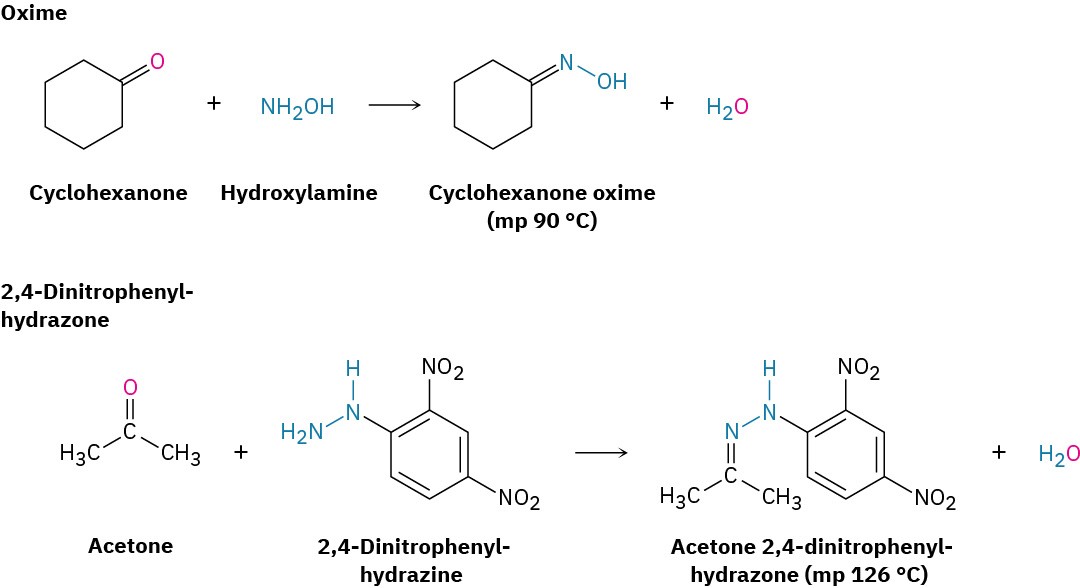
Imine formation with such reagents as hydroxylamine and 2,4-dinitrophenylhydrazine is sometimes useful because the products of these reactions—oximes and 2,4-dinitrophenylhydrazones (2,4-DNPs), respectively—are often crystalline and easy to handle. Such crystalline derivatives are occasionally prepared as a means of purifying and characterizing liquid ketones or aldehydes.
 Reaction of an aldehyde or ketone with a secondary amine, R2NH, rather than a primary amine yields an enamine. As shown in Figure 19.8, the process is identical to imine formation up to the iminium ion stage, but at this point there is no proton on nitrogen that can be lost to form a neutral imine product. Instead, a proton is lost from the neighboring carbon (the α carbon), yielding an enamine.
Reaction of an aldehyde or ketone with a secondary amine, R2NH, rather than a primary amine yields an enamine. As shown in Figure 19.8, the process is identical to imine formation up to the iminium ion stage, but at this point there is no proton on nitrogen that can be lost to form a neutral imine product. Instead, a proton is lost from the neighboring carbon (the α carbon), yielding an enamine.
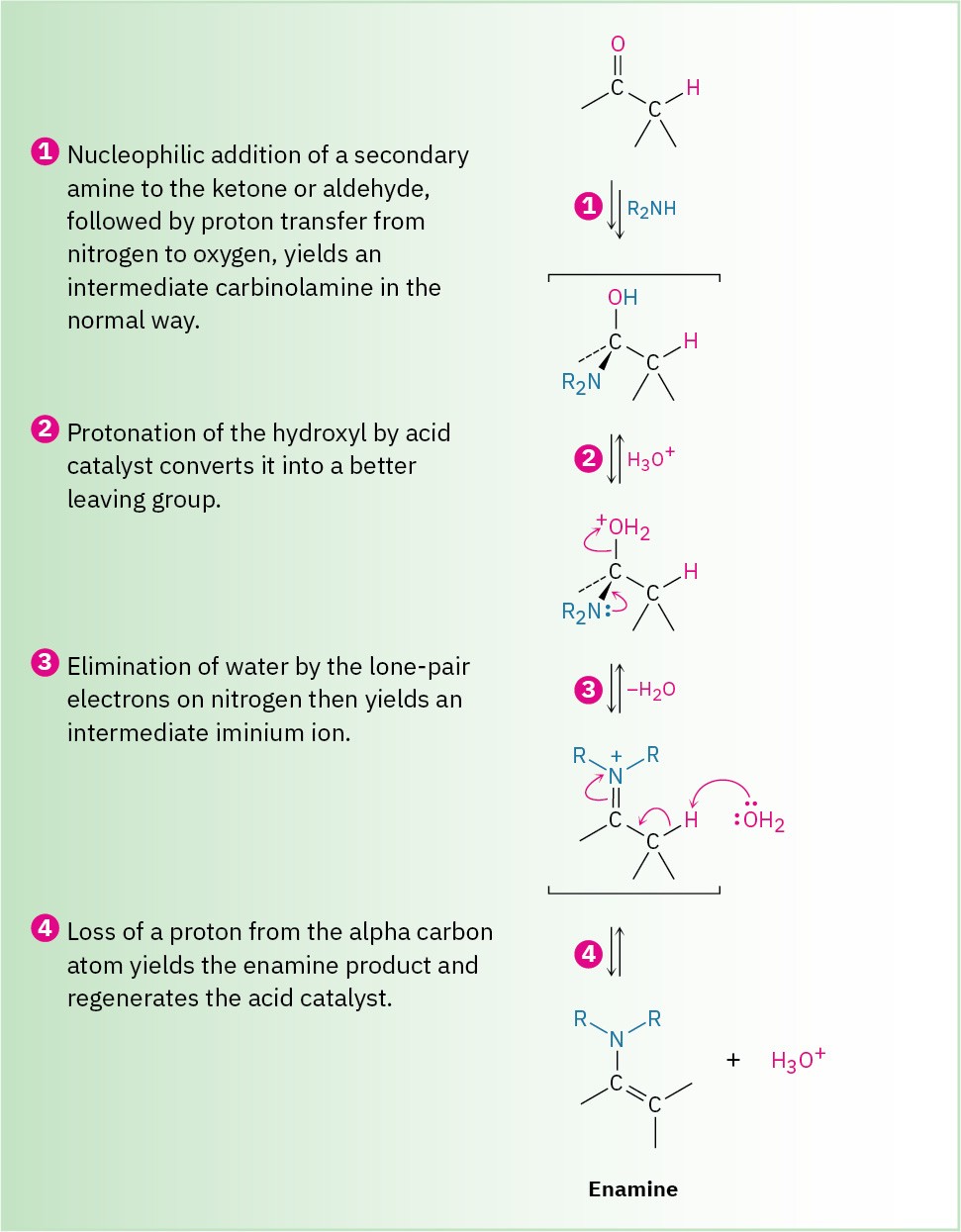
Figure 19.8 Mechanism for enamine formation by reaction of an aldehyde or ketone with a secondary amine, R2NH. The iminium ion intermediate formed in step 3 has no hydrogen attached to N and so must lose H+ from the carbon two atoms away.
Imine and enamine formation are slow at both high pH and low pH but reach a maximum rate at a weakly acidic pH around 4 to 5. For example, the profile of pH versus rate shown in Figure 19.9 for the reaction between acetone and hydroxylamine, NH2OH, indicates that the maximum reaction rate occurs at pH 4.5.
We can explain the observed pH dependence of imine formation by looking at the individual steps in the mechanism. As indicated in Figure 19.8, an acid catalyst is required in step 3 to protonate the intermediate carbinolamine, thereby converting the –OH into a better leaving group. Thus, reaction will be slow if not enough acid is present (that is, at high pH). On the other hand, if too much acid is present (low pH), the basic amine nucleophile is completely protonated, so the initial nucleophilic addition step can’t occur.
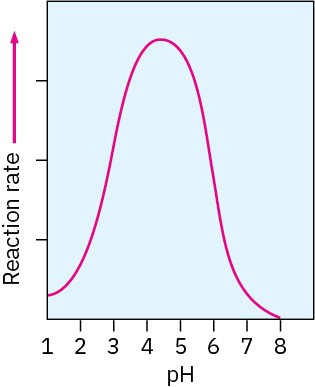 Figure 19.9 Dependence on pH of the rate of reaction between acetone and hydroxylamine: (CH3)2C═O + NH2OH → (CH3)2C═NOH + H2O.
Figure 19.9 Dependence on pH of the rate of reaction between acetone and hydroxylamine: (CH3)2C═O + NH2OH → (CH3)2C═NOH + H2O.
Evidently, a pH of 4.5 represents a compromise between the need for some acid to catalyze the rate-limiting dehydration step but not too much acid so as to avoid complete protonation of the amine. Each nucleophilic addition reaction has its own requirements, and conditions must be optimized to obtain maximum reaction rates.
Worked Example 19.1: Predicting the Product of Reaction between a Ketone and an Amine
Show the products you would obtain by acid-catalyzed reaction of 3-pentanone with methylamine, CH3NH2, and with dimethylamine, (CH3)2NH.
Strategy
An aldehyde or ketone reacts with a primary amine, RNH2, to yield an imine, in which the carbonyl oxygen atom has been replaced by the =N–R group of the amine. Reaction of the same aldehyde or ketone with a secondary amine, R2NH, yields an enamine, in which the oxygen atom has been replaced by the –NR2 group of the amine and the double bond has moved to a position between the former carbonyl carbon and the neighboring carbon.
Solution

Problem 19-10
Show the products you would obtain by acid-catalyzed reaction of cyclohexanone with ethylamine, CH3CH2NH2, and with diethylamine, (CH3CH2)2NH.
Problem 19-11
Imine formation is reversible. Show all the steps involved in the acid-catalyzed reaction of an imine with water (hydrolysis) to yield an aldehyde or ketone plus primary amine.
Problem 19-12
Draw the following molecule as a skeletal structure, and show how it can be prepared from a ketone and an amine.