Addition of Hydride Reagents: Reduction
We saw in Section 17.4 that the most common m Jethod for preparing alcohols, both in the laboratory and in living organisms, is by the reduction of carbonyl compounds. Aldehydes are reduced with sodium borohydride (NaBH4) to give primary alcohols, and ketones are reduced similarly to give secondary alcohols.
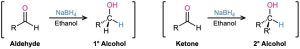
Carbonyl reduction occurs by a typical nucleophilic addition mechanism under basic conditions, as shown earlier in Figure 19.5a. Although the details of carbonyl-group reductions are complex, LiAlH4 and NaBH4 act as if they were donors of hydride ion nucleophile, :H–, and the initially formed alkoxide ion intermediate is then protonated by addition of aqueous acid. The reaction is effectively irreversible because the reverse process would require expulsion of a very poor leaving group.
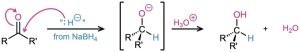
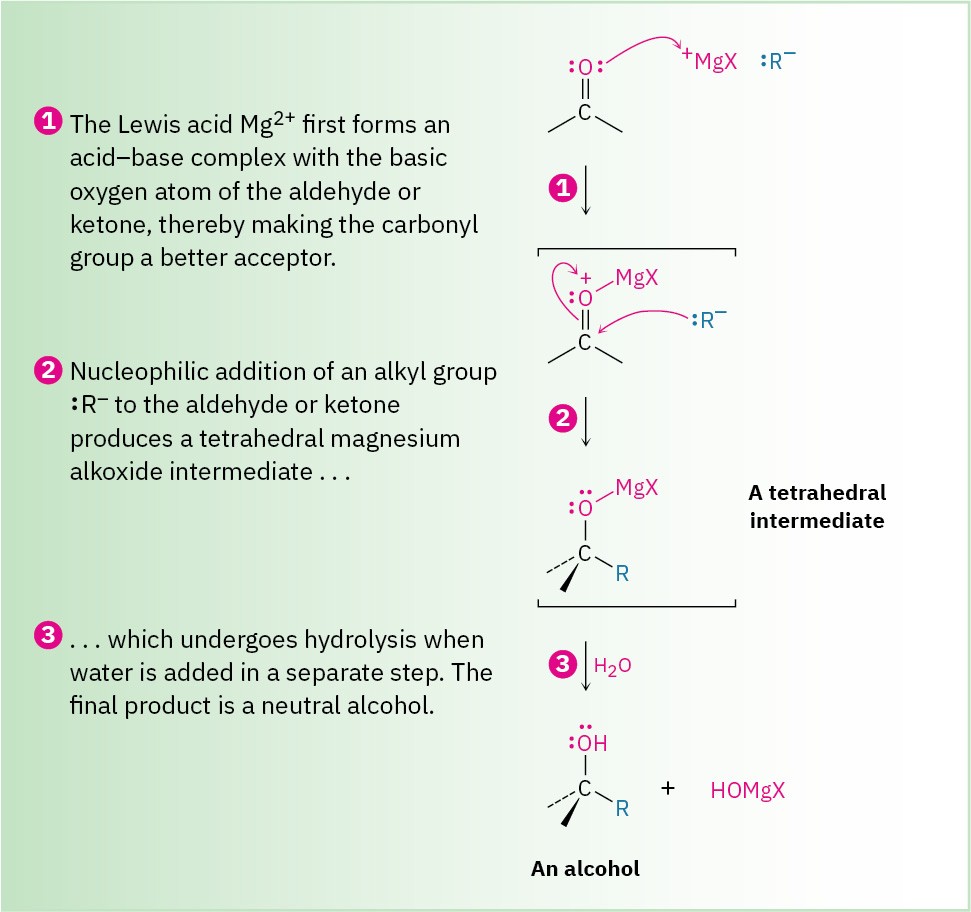
Figure 19.6 Mechanism of the Grignard reaction. Complexation of the carbonyl oxygen with the Lewis acid Mg2+ and subsequent nucleophilic addition of a carbanion to an aldehyde or ketone is followed by protonation of the alkoxide intermediate to yield an alcohol.
Addition of Grignard Reagents, RMgX
Just as aldehydes and ketones undergo nucleophilic addition with hydride ion to give alcohols, they undergo a similar addition with Grignard reagent nucleophiles, R:– +MgX. Aldehydes give secondary alcohols on reaction with Grignard reagents in ether solution, and ketones give tertiary alcohols.
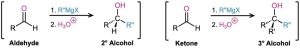
As shown in Figure 19.6, a Grignard reaction begins with an acid–base complexation of Mg2+ to the carbonyl oxygen atom of the aldehyde or ketone, thereby making the carbonyl group a better electrophile. Nucleophilic addition of R:– then produces a tetrahedral magnesium alkoxide intermediate, and protonation by addition of water or dilute aqueous acid in a separate step yields the neutral alcohol. Like reduction, Grignard additions are effectively irreversible because a carbanion is too poor a leaving group to be expelled in a reversal step.

