Aldehydes and ketones are converted into alkenes by means of a nucleophilic addition called the Wittig reaction. The reaction has no direct biological counterpart but is important both because of its wide use in drug manufacture and because of its mechanistic similarity to reactions of the coenzyme thiamin diphosphate, which we’ll see in Section 29.6.
In the Wittig reaction, a triphenylphosphorus ylide, R2C– PPh3 also called a phosphorane and sometimes written in the resonance form R2C═P(Ph)3, adds to an aldehyde or ketone to yield a four-membered cyclic intermediate called an oxaphosphetane. The oxaphosphetane is not isolated, but instead spontaneously decomposes to give an alkene plus triphenylphosphine oxide, O═PPh3. In effect, the oxygen atom of the aldehyde or ketone and the R2C= bonded to phosphorus exchange places. An ylide (pronounced ill-id—is) a neutral, dipolar compound with adjacent positive and negative charges.
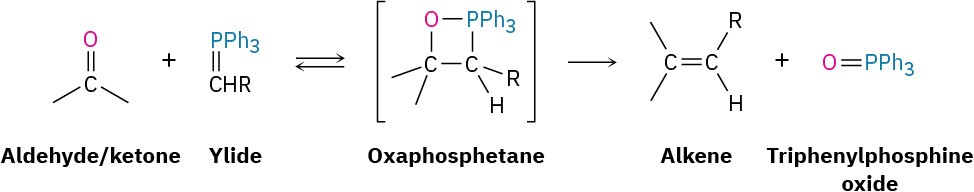
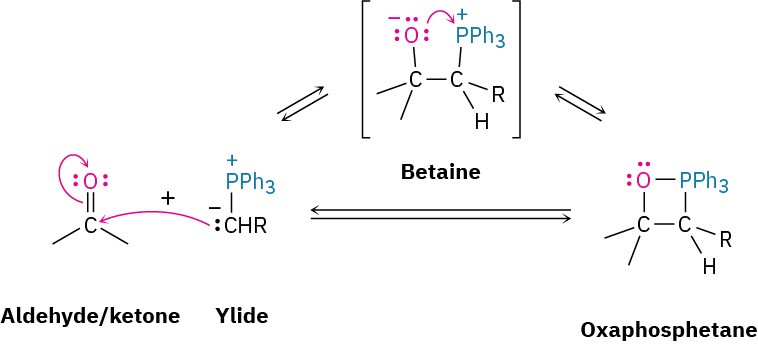
The initial addition takes place by different pathways depending on the structure of the reactants and the exact experimental conditions. One pathway involves a one-step cycloaddition process analogous to the Diels–Alder cycloaddition reaction (Section 14.4). The other pathway involves a nucleophilic addition reaction to give a dipolar intermediate called a betaine (bay-ta-een), which undergoes ring closure.
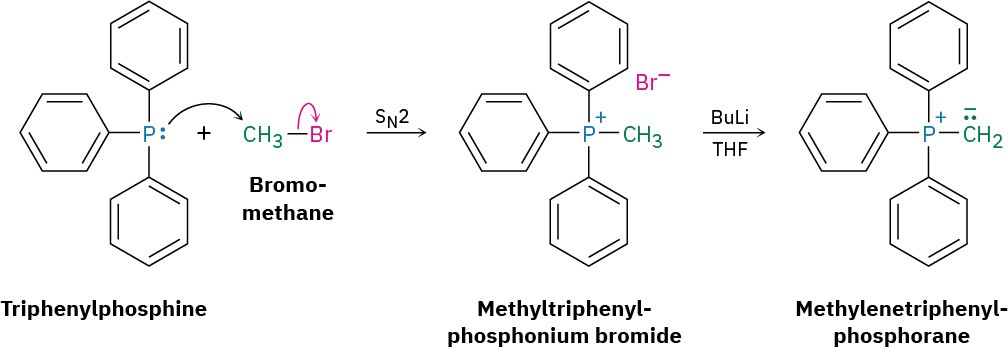
The phosphorus ylides necessary for Wittig reactions are easily prepared by SN2 reaction of primary (and some secondary) alkyl halides with triphenylphosphine, (Ph)3P, followed by treatment with base. Triphenylphosphine is a good nucleophile in SN2 reactions, and yields of the resultant alkyltriphenylphosphonium salts are high. Because of the positive charge on phosphorus, the hydrogen on the neighboring carbon is weakly acidic and can be removed by a strong base such as butyllithium (BuLi) to generate the neutral ylide. For example:
The Wittig reaction is extremely general, and a great many monosubstituted, disubstituted, and trisubstituted alkenes can be prepared from the appropriate combination of phosphorane and aldehyde or ketone. Tetrasubstituted alkenes, however, can’t be prepared this way because of steric hindrance.
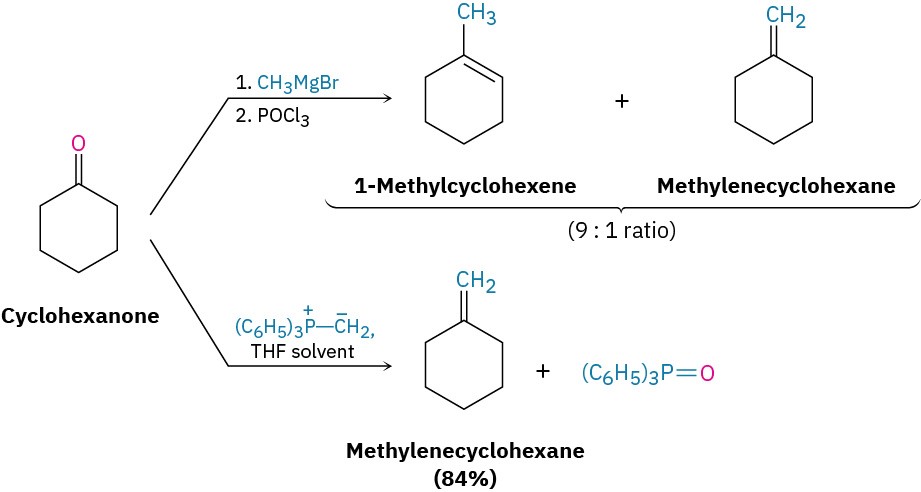
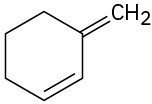
The real value of the Wittig reaction is that it yields a pure alkene of predictable structure. The C═C bond in the product is always exactly where the C═O group was in the reactant, and no alkene isomers (except E,Z isomers) are formed. For example, Wittig reaction of cyclohexanone with methylenetriphenylphosphorane yields only the single alkene product methylenecyclohexane. By contrast, addition of methylmagnesium bromide to cyclohexanone, followed by dehydration with POCl3, yields a roughly 9 : 1 mixture of two alkenes.
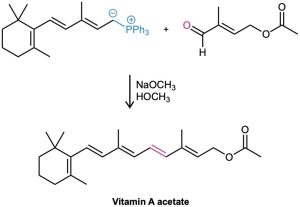
Wittig reactions are used commercially in the synthesis of numerous pharmaceutical products. For example, the German chemical company BASF prepares vitamin A by using a Wittig reaction between a 15-carbon ylide and a 5-carbon aldehyde.
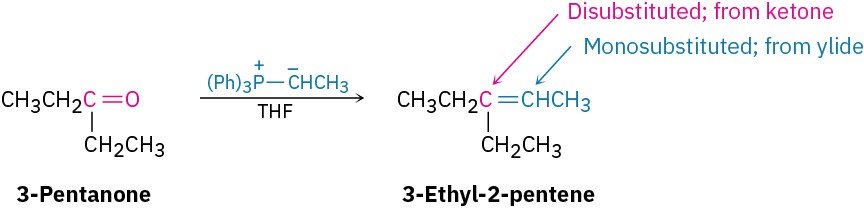
Worked Example 19.3: Synthesizing an alkene using a Wittig reaction
What carbonyl compound and what phosphorus ylide might you use to prepare 3-ethyl-2- pentene?
Strategy
An aldehyde or ketone reacts with a phosphorus ylide to yield an alkene in which the oxygen atom of the carbonyl reactant is replaced by the ═CR2 of the ylide. Preparation of the phosphorus ylide itself usually involves SN2 reaction of a primary alkyl halide with triphenylphosphine, so the ylide is typically primary, RCH═P(Ph)3. This means that the disubstituted alkene carbon in the product comes from the carbonyl reactant, while the monosubstituted alkene carbon comes from the ylide.
Solution
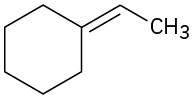
Problem 19-16
What carbonyl compound and what phosphorus ylide might you use to prepare each of the following compounds?
(a)
(b)
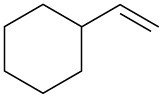
(c)
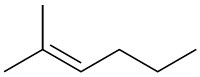
(d)
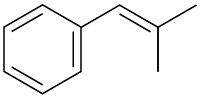
(e)
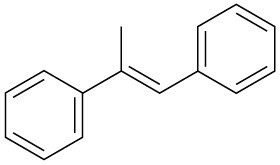
(f)
Problem 19-17
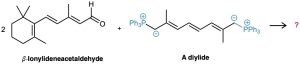
β-Carotene, a yellow food-coloring agent and dietary source of vitamin A can be prepared by a double Wittig reaction between 2 equivalents of β-ionylideneacetaldehyde and a diylide. Show the structure of the β-carotene product.