Like the word reduction used in the previous section for the addition of hydrogen to a double bond, the word oxidation has a slightly different meaning in organic chemistry than what you might have previously learned. In general chemistry, an oxidation is defined as the loss of one or more electrons by an atom. In organic chemistry, however, an oxidation is a reaction that results in a loss of electron density for carbon, caused either by bond formation between carbon and a more electronegative atom—usually oxygen, nitrogen, or a halogen—or by bond-breaking between carbon and a less electronegative atom—usually hydrogen. Note that an oxidation often adds oxygen, while a reduction often adds hydrogen.

In the laboratory, alkenes are oxidized to give epoxides on treatment with a peroxyacid, RCO3H, such as meta-chloroperoxybenzoic acid. An epoxide, also called an oxirane, is a cyclic ether with an oxygen atom in a three-membered ring. For example:
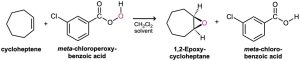
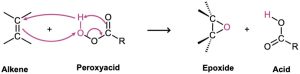
Peroxyacids transfer an oxygen atom to the alkene with syn stereochemistry—both C−O bonds form on the same face of the double bond—through a one-step mechanism without intermediates. The oxygen atom farthest from the carbonyl group is the one transferred.
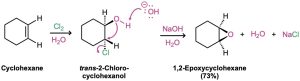
Another method for the synthesis of epoxides involves the use of halohydrins, prepared by electrophilic addition of HO−X to alkenes (Section 8.3). When a halohydrin is treated with base, HX is eliminated and an epoxide is produced.
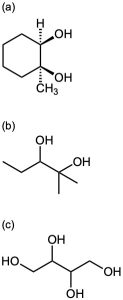
Epoxides undergo an acid-catalyzed ring-opening reaction with water (a hydrolysis) to give the corresponding 1,2-dialcohol, or diol, also called a glycol. Thus, the net result of the two- step alkene epoxidation/hydrolysis is hydroxylation—the addition of an −OH group to each of the two double-bond carbons. In fact, approximately 204 million tons of ethylene glycol, HOCH2CH2OH, most of it used for automobile antifreeze, are produced worldwide each year by the epoxidation of ethylene and subsequent hydrolysis.
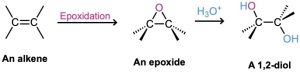
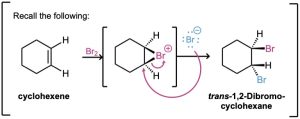
Acid-catalyzed epoxide opening begins with protonation of the epoxide to increase its reactivity, followed by nucleophilic addition of water. This nucleophilic addition is analogous to the final step of alkene bromination, in which a cyclic bromonium ion is opened by a nucleophile (Section 8.2). That is, a trans-1,2-diol results when an epoxycycloalkane is opened by aqueous acid, just as a trans-1,2-dibromide results when a cycloalkene is brominated. We’ll look at epoxide chemistry in more detail in Section 18.6.

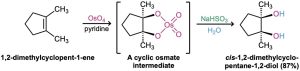
Hydroxylation can also be carried out directly (without going through an intermediate epoxide) by treating an alkene with osmium tetroxide, OsO4. The reaction occurs with syn stereochemistry and does not involve a carbocation intermediate. Instead, it takes place through an intermediate cyclic osmate, which is formed in a single step by addition of OsO4 to the alkene. This cyclic osmate is then cleaved using aqueous sodium bisulfite, NaHSO3.
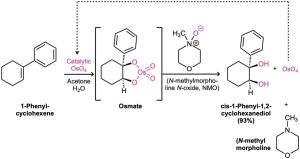
Because OsO4 is both very expensive and very toxic, the reaction is usually carried out using only a small, catalytic amount of OsO4 in the presence of a stoichiometric amount of a safe and inexpensive co-oxidant such as N-methylmorpholine N-oxide, abbreviated NMO. The initially formed osmate intermediate reacts rapidly with NMO to yield the product diol plus N-methylmorpholine and reoxidized OsO4, which reacts with more alkene in a catalytic cycle.
Problem 8-13
What product would you expect from reaction of cis-2-butene with mCPBA? Show the stereochemistry.
Problem 8-14
Starting with an alkene, how would you prepare each of the following compounds?