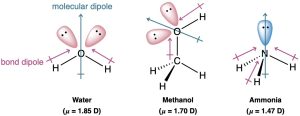
Just as individual bonds can be polar, molecules as a whole can be polar. Molecular polarity results from the vector summation of all individual bond dipoles in the molecule. This net polarity is measured and defined the dipole moment, often shown as μ (lowercase Greek letter mu). The larger the value, the larger the overall molecular dipole. Water (μ = 1.85 D), methanol (μ = 1.70 D), and ammonia (μ = 1.47 D), have substantial dipole moments, characteristic of polar molecules.
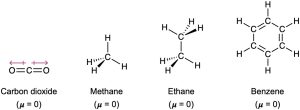
In contrast, non-polar molecules have a dipole moment of zero. In the case of carbon dioxide, this is because its two bond dipoles are of equal strength and oriented in opposing directions, resulting in a net vector summation of zero. In the case of methane, ethane, and benzene, these molecules are comprised of solely C-C covalent bonds and C-H covalent bonds. Because there are no polar covalent bonds in these molecules, these molecules are nonpolar.
Problem 2-5
Ethylene glycol, HOCH2CH2OH possesses an internal hydrogen bond between the two –OH groups, resulting in a dipole moment. Explain.
Problem 2-6
Make three-dimensional drawings of the following molecules, and predict whether each is polar.
(a) H2C=CH2
(b) CHCl3
(c) CH2Cl2
(d) H2C=CCl2

