When an amine reacts with an acid chloride, an amide is formed. What would happen, though, if a diamine and a diacid chloride were allowed to react? Each partner would form two amide bonds, linking more and more molecules together until a giant polyamide resulted. In the same way, reaction of a diol with a diacid would lead to a polyester.
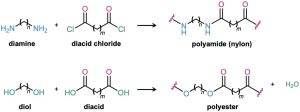
There are two main classes of synthetic polymers: chain-growth polymers and step-growth polymers. The polyethylene and other alkene and diene polymers that we saw in Section 8.10 and Section 14.6 are called chain-growth polymers because they are produced in chain-reaction processes. An initiator adds to a C═C bond to give a reactive intermediate, which adds to a second alkene molecule to produce a new intermediate, which adds to a third molecule, and so on. By contrast, polyamides and polyesters are step-growth polymers because each bond in the polymer is formed independently in a discrete step, often the nucleophilic acyl substitution reaction of a carboxylic acid derivative. Some commercially important step-growth polymers are shown in Table 21.2.
Table 21.2 Some Common Step-Growth Polymers and Their Uses
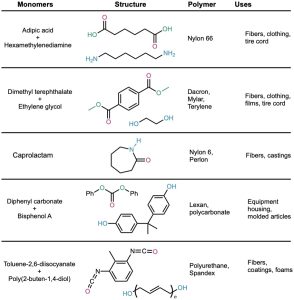
Polyamides (Nylons)
The best known step-growth polymers are the polyamides, or nylons, first prepared in 1930 by Wallace Carothers at the DuPont Company by heating a diamine with a diacid. For instance, nylon 66 is prepared by reaction of adipic acid (hexanedioic acid) with hexamethylenediamine (1,6-hexanediamine) at 280 °C. The designation “66” indicates the number of carbon atoms in the diamine (the first 6) and the diacid (the second 6).
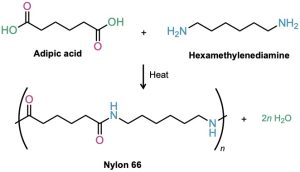
Nylons are used both in engineering applications and in making fibers. A combination of high impact strength and abrasion resistance makes nylon an excellent metal substitute for bearings and gears. As fiber, nylon is used in a variety of applications, from clothing to tire cord to ropes.
Polyesters
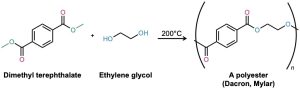
The most generally useful polyester is made by reaction between dimethyl terephthalate (dimethyl 1,4-benzenedicarboxylate) and ethylene glycol (1,2-ethanediol). The product is used under the trade name Dacron to make clothing fiber or tire cord and under the name Mylar to make recording tape. The tensile strength of poly(ethylene terephthalate) film is nearly equal to that of steel.
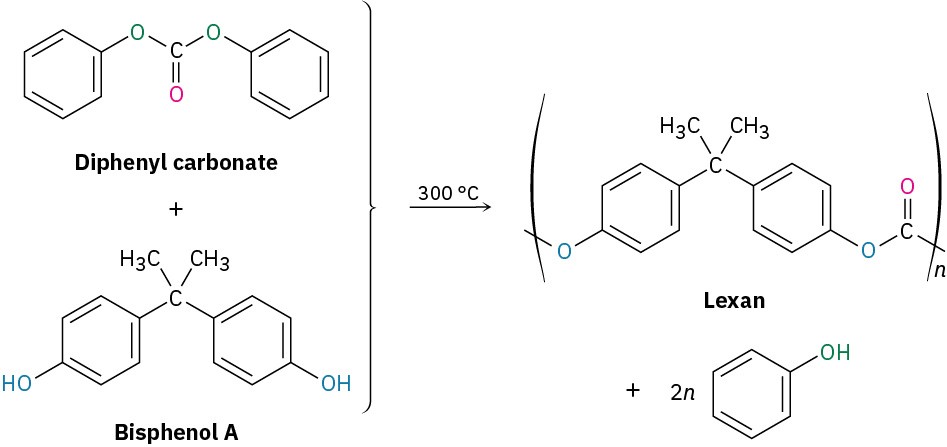
Lexan, a polycarbonate prepared from diphenyl carbonate and bisphenol A, is another commercially valuable polyester. Lexan has an unusually high impact strength, making it valuable for use in bicycle helmets and laptop cases.
Sutures and Biodegradable Polymers
Because plastics are too often thrown away rather than recycled, much work has been carried out on developing biodegradable polymers, which can be broken down rapidly in landfills by soil microorganisms. Among the most common biodegradable polymers are poly(glycolic acid) (PGA), poly(lactic acid) (PLA), and poly(hydroxybutyrate) (PHB). All are polyesters and are therefore susceptible to hydrolysis of their ester links. Copolymers of PGA with PLA have found a particularly wide range of uses. A 90/10 copolymer of poly(glycolic acid) with poly(lactic acid) is used to make absorbable sutures, for instance. The sutures are entirely hydrolyzed and absorbed by the body within 90 days after surgery.
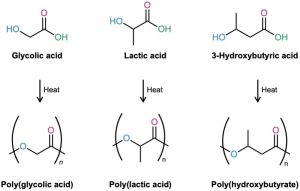
In Europe, interest has centered particularly on poly(hydroxybutyrate), which can be made into films for packaging as well as into molded items. The polymer degrades within four weeks in landfills, both by ester hydrolysis and by an E1cB elimination reaction of the oxygen atom β to the carbonyl group. The use of poly(hydroxybutyrate) is limited at present by its cost—about four times that of polypropylene.
Problem 21-23
Draw structures of the step-growth polymers you would expect to obtain from the following reactions:
(a)
![]()
(b)
![]()
(c)
![]()
Problem 21-24
Kevlar, a nylon polymer prepared by reaction of 1,4-benzenedicarboxylic acid (terephthalic acid) with 1,4-benzenediamine (p-phenylenediamine), is so strong that it’s used to make bulletproof vests. Draw the structure of a segment of Kevlar.

